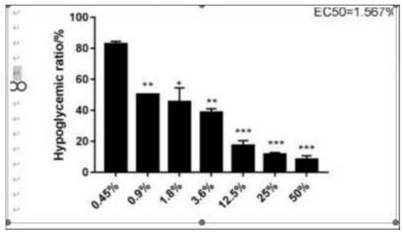
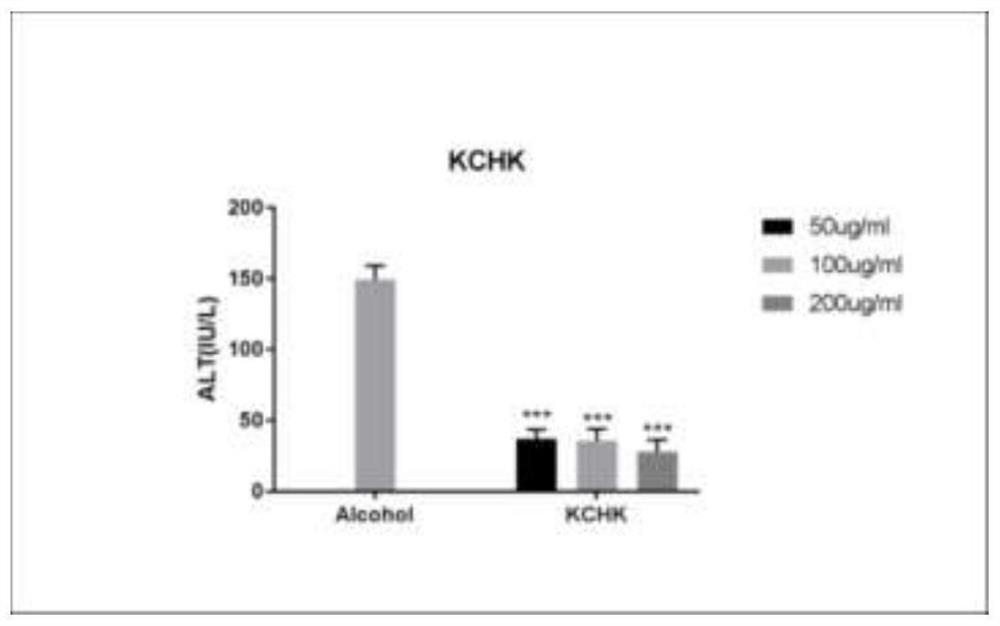
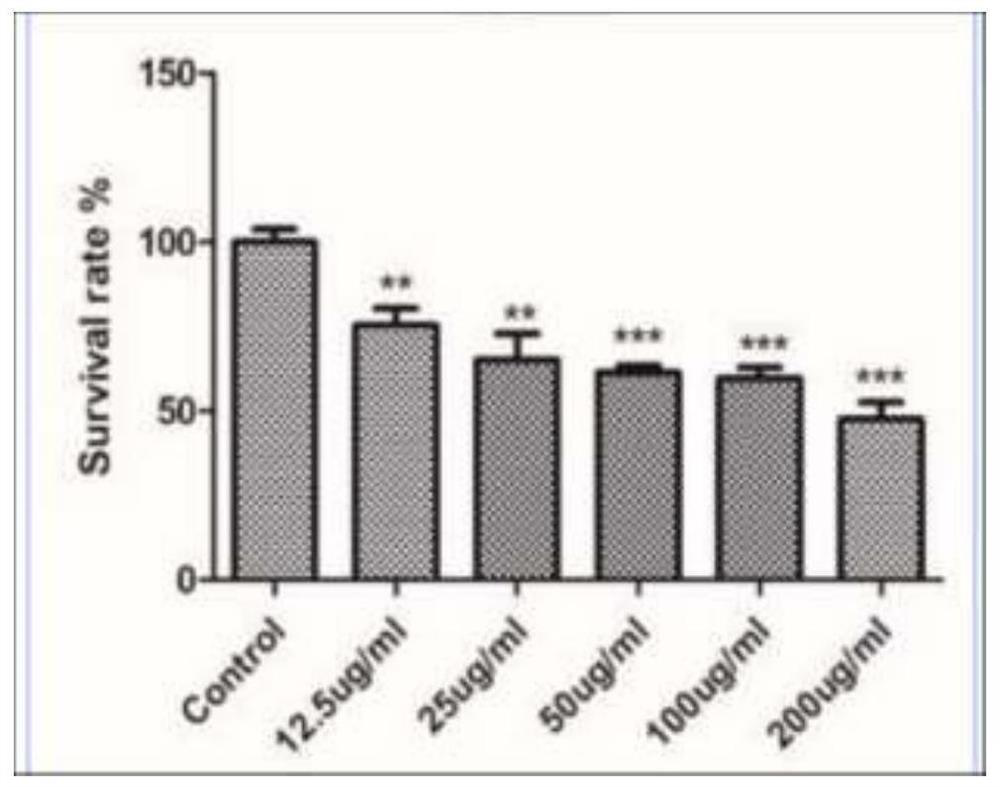
Manufacturing of medical fulvic acid standard substance and application of medical fulvic acid standard substance in cell experiment
A technology of fulvic acid and standard products, which can be used in the detection of programmed cell death, drug screening, and compound screening. Effects of health, value-added enhancement, and economic prosperity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Get 100 parts of lignite raw materials, after screening and pulverizing, at 130° C. of reactor temperature, under reaction pressure 0.13 MPa, adopt concentration to be 0.05% (mass) of raw material after sodium percarbonate, calcium percarbonate catalytic oxidation reaction 3h, in belt sieve plate The first-stage precipitation separation is carried out in the reactor to remove the insoluble impurities of pharmaceutical fulvic acid; then the FX100370X1070 vertical cyclone, the LW255X765-NLWX020-N decanter screw separator is used for the second-stage separation, and the DHC300 disc centrifuge is used for the third-stage separation. High-grade liquid-solid separation to remove insoluble fine impurities in pharmaceutical fulvic acid; using 0.05% pharmaceutical grade chitosan (provided by the Chemistry and Molecular Engineering Research Office of East China University of Science and Technology), at a temperature of 50°C, for 1 hour, and a pressure of 0.12MPa Carry out gel hydr...
Embodiment 2
[0029]Take 100 parts of peat raw material, after screening and pulverizing, at a reaction temperature of 130°C and a reaction pressure of 0.13 MPa, use sodium percarbonate and calcium percarbonate with a concentration of 0.01% (mass) of the raw material to catalyze the oxidation reaction for 3 hours, and then carry out primary precipitation separation , to remove insoluble coarse impurities; then use FX100370X1070 vertical cyclone machine, LW255X765-NLWX020-N horizontal screw machine for liquid-solid separation, and DHC300 disc machine for re-separation to remove insoluble fine impurities in fulvic acid; use 0.05% pharmaceutical Grade chitosan (provided by the Chemistry and Molecular Engineering Research Office of East China University of Science and Technology), at a temperature of 50 ° C, for 1 hour, and a pressure of 0.12 MPa for deashing by gel hydrolysis phase transition method, after the effective rate reaches 96.0-98.5%, it is crude Fulvic acid pharmaceuticals, at a temp...
Embodiment 3
[0031] Get 100 parts of weathered lignite raw materials, after screening and pulverizing, at a reactor temperature of 130° C., under a reaction pressure of 0.13 MPa, adopt a concentration of 0.1% (mass) of raw materials for sodium percarbonate and calcium percarbonate to catalyze the oxidation reaction for 3 hours, and then use a sieve The first-stage precipitation separation is carried out in the reactor of the plate to remove the insoluble impurities of pharmaceutical fulvic acid; then the FX100370X1070 vertical cyclone, the LW255X765-NLWX020-N decanter screw separator is used for the second-stage separation, and the DHC300 disc centrifuge is used for further separation. Three-stage liquid-solid separation to remove insoluble fine impurities in pharmaceutical fulvic acid; using 0.05% pharmaceutical grade chitosan (provided by the Chemistry and Molecular Engineering Research Office of East China University of Science and Technology), at a temperature of 50°C, for 1 hour, and a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com