Ticagrelor pharmaceutical composition as well as preparation method and application thereof
A technology for ticagrelor and a composition, which is applied in the field of ticagrelor pharmaceutical compositions and preparation thereof, can solve the problems of increasing production costs, being unfavorable for ensuring the quality uniformity, effectiveness and safety of pharmaceutical preparations, and achieving Ease of operation, shortened production cycle and improved quality uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-3
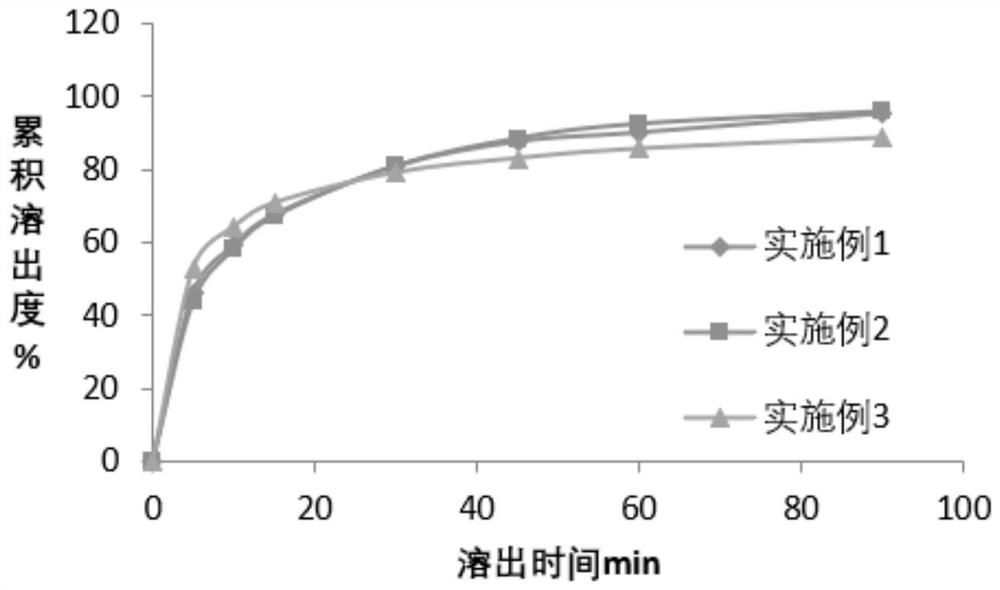
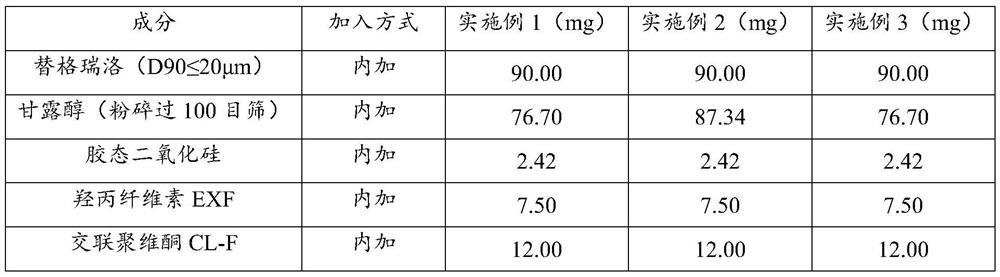
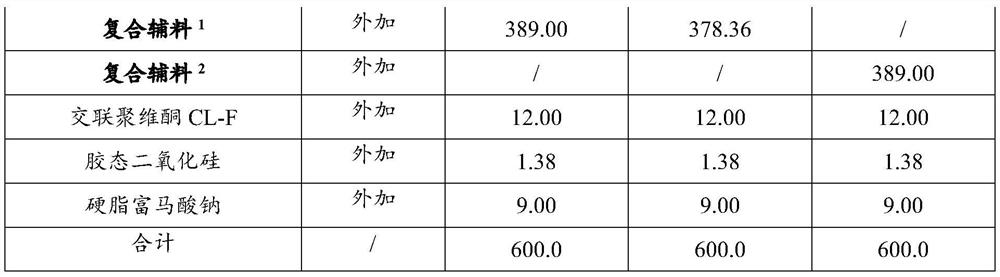
[0088] Example 1-3 Preparation of Ticagrelor Tablets
[0089] The composition of the ticagrelor tablet of embodiment 1-3 is shown in Table 1, and its preparation method comprises the following steps:
[0090] (1) Pretreatment of added raw and auxiliary materials
[0091] Weigh the prescribed amount of ticagrelor (D90≤20μm), mannitol (crushed through a 100-mesh sieve), colloidal silicon dioxide (inside), hyprolose EXF, crospovidone CL-F (inside ), put it in a ziplock bag, manually mix for 3 minutes, pass through a 40-mesh sieve, and set aside.
[0092] (2) Granulation:
[0093] Put the pretreated internally added raw and auxiliary materials in the wet mixing granulator, the stirring speed is 400rpm, the shearing speed is 800rpm, and the equipment is turned on for 5min.
[0094] Weigh purified water, under the starting state (stirring speed 400rpm, shearing speed 800rpm), slowly (20 ~ 30s) add purified water to make soft material, after adding, continue stirring and shearin...
Embodiment 1
[0122] Table 4 embodiment 1, embodiment 3 tablet stability
[0123]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com