High-entropy Prussian blue material and preparation method thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] This embodiment provides a method for preparing a high-entropy Prussian blue-like sodium-ion battery cathode material, comprising the following steps:
[0049] Dissolve 3 mol of sodium ferrocyanide decahydrate, 10 g of polyvinylpyrrolidone, and 2 mol of sodium citrate in a mixed solvent consisting of 1 L of deionized water and 100 mL of absolute ethanol to obtain a precursor solution A;
[0050]Dissolve 0.3 mol manganese sulfate, 0.3 mol copper sulfate, 0.3 mol nickel acetate, 0.3 mol cobalt chloride, 0.3 mol ferric nitrate and 2 mol sodium citrate in a mixed solvent composed of 1L deionized water and 100mL absolute ethanol to obtain a precursor solution B;
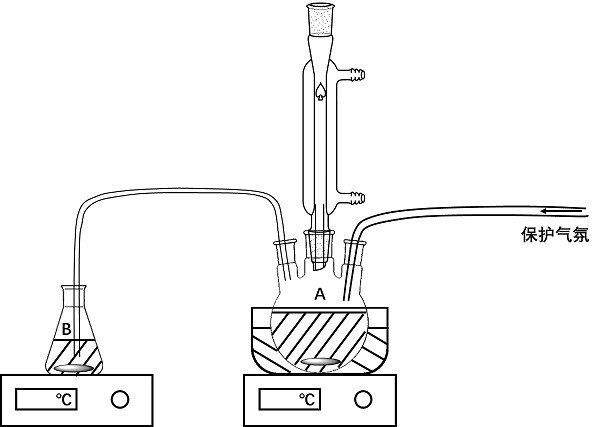
[0051] use figure 1 The device shown is for co-precipitation reaction, wherein container A contains precursor solution A, container B contains precursor solution B, and the precursor solution B is slowly dripped into container A at a rate of 1 mL / min through a silicone tube using a peristaltic pump , and the temp...
Embodiment 2
[0060] This embodiment provides a method for preparing a high-entropy Prussian blue-like sodium-ion battery cathode material, comprising the following steps:
[0061] 4mol sodium ferrocyanide decahydrate, 20g cetyltrimethylammonium bromide, and 1mol1-10-phenanthroline were dissolved in a mixed solvent composed of 1L deionized water and 300mL anhydrous n-butanol, Obtain precursor solution A;
[0062] Dissolve 0.4mol manganese chloride, 0.4mol copper oxalate, 0.4mol nickel acetate, 0.4mol ethylenediaminetetraacetic acid cobalt, 0.4mol ferric nitrate, 0.4mol titanium chloride and 1mol 1-10-phenanthroline in 1L deionized Precursor solution B is obtained in a mixed solvent composed of water;
[0063] use figure 1 The device shown is for co-precipitation reaction, wherein container A contains precursor solution A, container B contains precursor solution B, and the precursor solution B is slowly added dropwise to container A at a rate of 5 mL / min through a silicone tube using a per...
Embodiment 3
[0071] This embodiment provides a method for preparing a high-entropy Prussian blue-like sodium-ion battery cathode material, comprising the following steps:
[0072] Dissolve 1mol of sodium ferrocyanide decahydrate, 300g of sodium dodecylbenzenesulfonate, and 0.1mol of disodium edetate in a mixed solvent consisting of 1L of deionized water and 100mL of anhydrous methanol to obtain a precursor solution A;
[0073] Dissolve 0.2mol manganese sulfate, 0.1mol copper sulfate, 0.1mol nickel acetate, 0.2mol titanium chloride, 0.2mol vanadium chloride, 0.2mol chromium chloride and 0.001mol edetate disodium in 1L deionized water and Precursor solution B was obtained in a mixed solvent composed of 500mL absolute ethanol;
[0074] use figure 1 The device shown is for co-precipitation reaction, wherein container A contains precursor solution A, container B contains precursor solution B, and uses a peristaltic pump to slowly drop precursor solution B to container A at a rate of 50 mL / min...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com