Dual-response dipeptide supramolecular polymer, and preparation method and application thereof
A supramolecular polymer and dual response technology, which is applied to the preparation method of peptides, non-effective ingredients of polymer compounds, chemical instruments and methods, etc., can solve the problems of small synthesis dosage, long polypeptide chain and high synthesis cost, and achieve the goal of preparation Simple, Fluorescent Intensity Enhanced Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0080] The present invention also provides a preparation method of the supramolecular monomer, comprising the following steps:
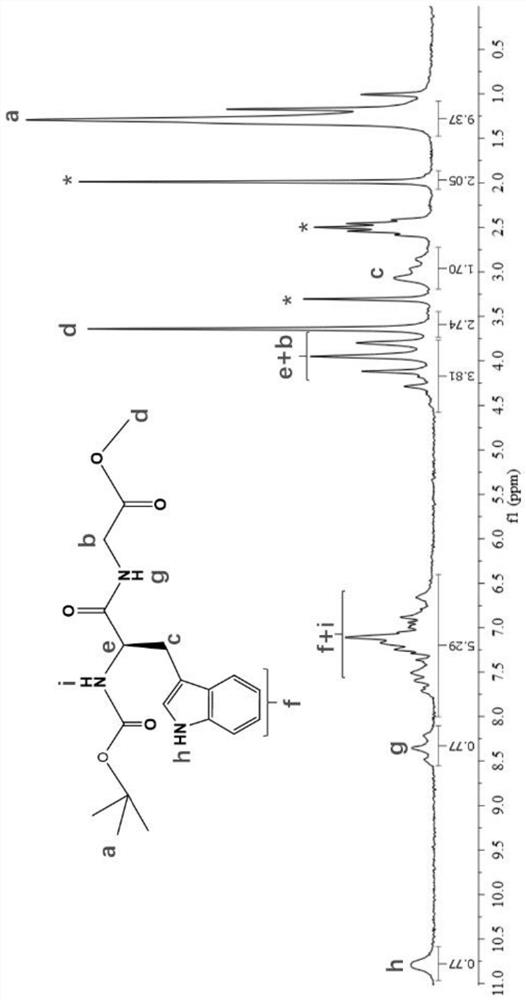
[0081] (1) Tryptophan, 1-hydroxybenzotriazole, glycine methyl ester hydrochloride, dichloromethane, N,N-diisopropylethylamine and 1-ethyl-(3-dimethylamino Propyl) carbodiimide hydrochloride mixed, carry out condensation reaction, obtain compound A;
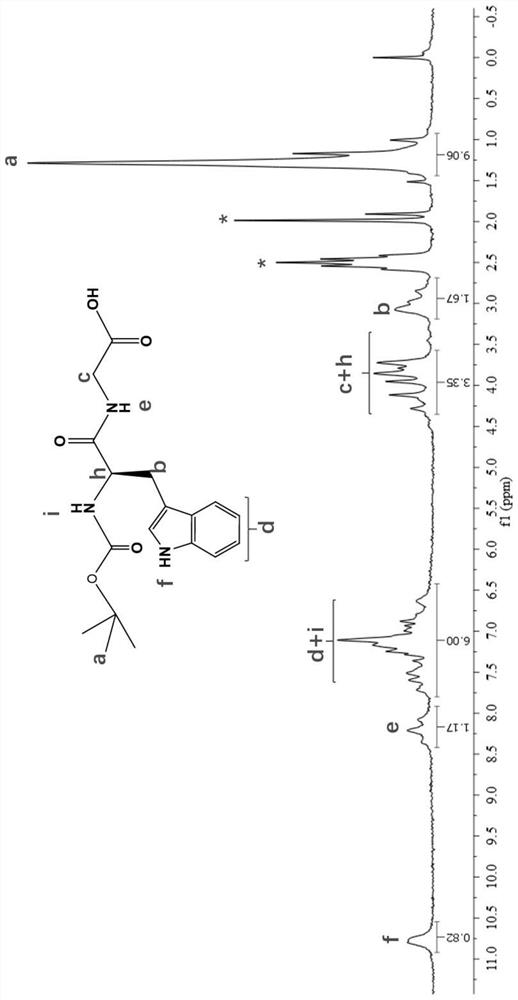
[0082] (2) Mix compound A, hydrated lithium hydroxide and methanol aqueous solution, and carry out a substitution reaction to obtain compound B;
[0083] (3) Mix compound B, branched alkoxy ether, dichloromethane, 4-dimethylaminopyridine and 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride, and carry out Esterification reaction to obtain compound C;
[0084] (4) Compound C, dichloromethane and trifluoroacetic acid were mixed for a substitution reaction to obtain compound D;
[0085] (5) Under alkaline conditions, compound D, monomers and reactants are mixed for amidation reaction to obtain t...
Embodiment 1
[0153] In a nitrogen atmosphere, mix 1 mmol of Boc-L-tryptophan, 1.2 mmol of 1-hydroxybenzotriazole, 1.1 mmol of glycine methyl ester hydrochloride and 10 mL of dichloromethane, and then add 2 mmol of N, N-diisopropylethylamine was initially reacted at 0°C and 350rpm for 20 minutes to obtain a reaction system, and the reaction system was mixed with 1.2 mmol of 1-ethyl-(3-dimethylaminopropyl)carbodiimide salt acid salt mixture, nitrogen replacement, secondary reaction at 0°C, 350rpm for 20min; after the secondary reaction, condensation reaction at 25°C for 11h; TLC detection after the reaction, the volume ratio of petroleum ether and ethyl acetate is 2 : 3, then use 10% potassium bisulfate solution to extract, the volume ratio of reaction solution and potassium bisulfate solution is 1:1.5; then use dichloromethane to extract 3 times, the volume ratio of reaction solution and dichloromethane is 1 : 1.3; the combined total volume of the extracted organic phases is 13mL, and dried...
Embodiment 2
[0164] In a nitrogen atmosphere, mix 1.2 mmol of Boc-L-tryptophan, 1 mmol of 1-hydroxybenzotriazole, 1.3 mmol of glycine methyl ester hydrochloride and 4 mL of dichloromethane, and then add 2.6 mmol of N ,N-diisopropylethylamine was initially reacted at 0°C and 400rpm for 25min to obtain a reaction system, and the reaction system was mixed with 1.44mmol of 1-ethyl-(3-dimethylaminopropyl)carbodiimide Mix hydrochloride, replace nitrogen, and perform a secondary reaction at 0°C and 400rpm for 25 minutes; after the secondary reaction, condense at 30°C for 12 hours; after the reaction, perform TLC detection, the volume ratio of petroleum ether and ethyl acetate is 2.5:3.5, then adopt 10% potassium hydrogensulfate solution to extract, the volume ratio of reaction solution and potassium hydrogensulfate solution is 1:1.3; Then use dichloromethane to extract 3 times, the volume ratio of reaction solution and dichloromethane is 1:1.4; Combine the extracted organic phases and dry them wi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com