Method for preparing antigen binding units
A technology for combining units and antigens, applied in chemical instruments and methods, antiviral immunoglobulins, cells modified by introducing foreign genetic material, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0229] Example 1: Isolation and enrichment of B cells
[0230] Blood was collected from persons infected with SARS-CoV-2 who were cured and discharged (provided by Beijing You'an Hospital). The discharge criteria were (1) body temperature returned to normal for more than three days; (2) respiratory symptoms relieved; and (3) two consecutive sputum SARS-CoV-2 RT-PCR tests at one-day sampling intervals with negative results.
[0231] PBMC Cell Harvesting and B Cell Enrichment: Using STEMCELLSepMate in a P2+ Biosafety Laboratory TM -15 (Stemcell Technologies, catalog number: 86415) for extraction of PBMCs. Subsequently, the extracted PBMCs were enriched for memory B cells using the STEMCELL EasySep Human Memory B Cell Isolation Kit (Stemcell Technologies, catalog number: 17864) according to the manufacturer's instructions.
[0232] CD27+ memory B cell enrichment: According to the manufacturer's instructions, using STEMCELL EasySep HumanMemory B Cell Isolation Kit (Stemcell Tech...
Embodiment 2
[0234] Example 2: Acquisition and Identification of Antigen Binding Unit Sequence
[0235] According to the manufacturer's instructions, Chromium Single Cell V(D)J Reagent Kits (purchased from 10X genomics, catalog number: 100006) were used to perform single-cell transcriptome VDJ sequencing on the above-mentioned enriched memory B cells. The enriched B cells from 10 patients were regarded as a batch, and a total of six batches of sequencing analysis were performed.
[0236] Data were processed using the 10X Genomics CellRanger (3.1.0) pipeline. Reads generated from 5' gene expression profiling were aligned to the GRCh38 genome to generate a matrix of signature barcodes. Genes expressed in more than 10 cells were selected and cells were filtered based on gene number and percentage of mitochondrial genes to remove possible doublets. Cell types were identified using SingleR (Aran et al., 2019) against the Human Immunity Reference Dataset (see Monaco et al., 2019). Figure 7 S...
Embodiment 3
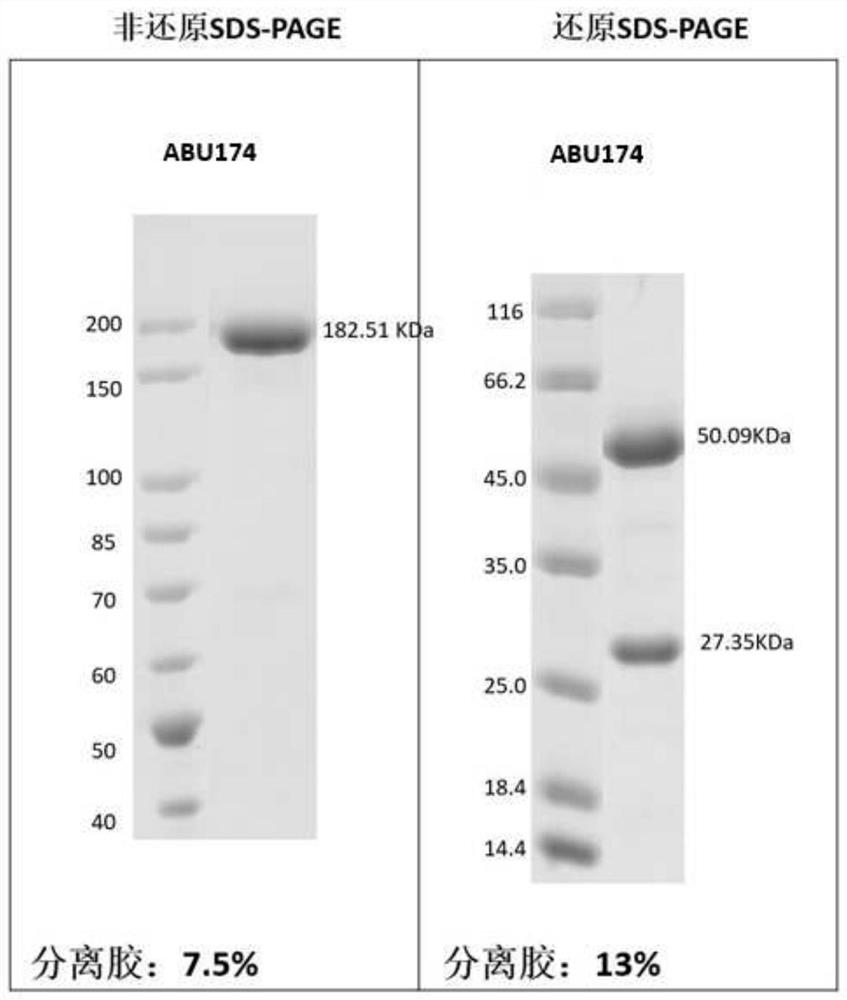
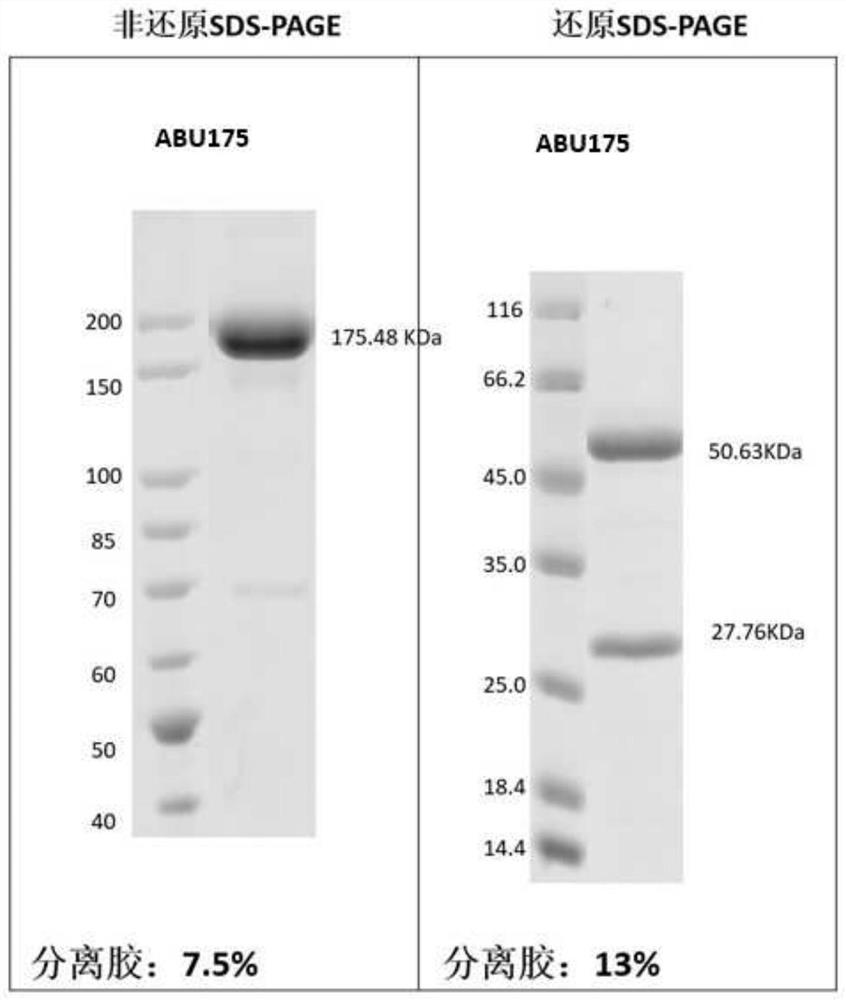
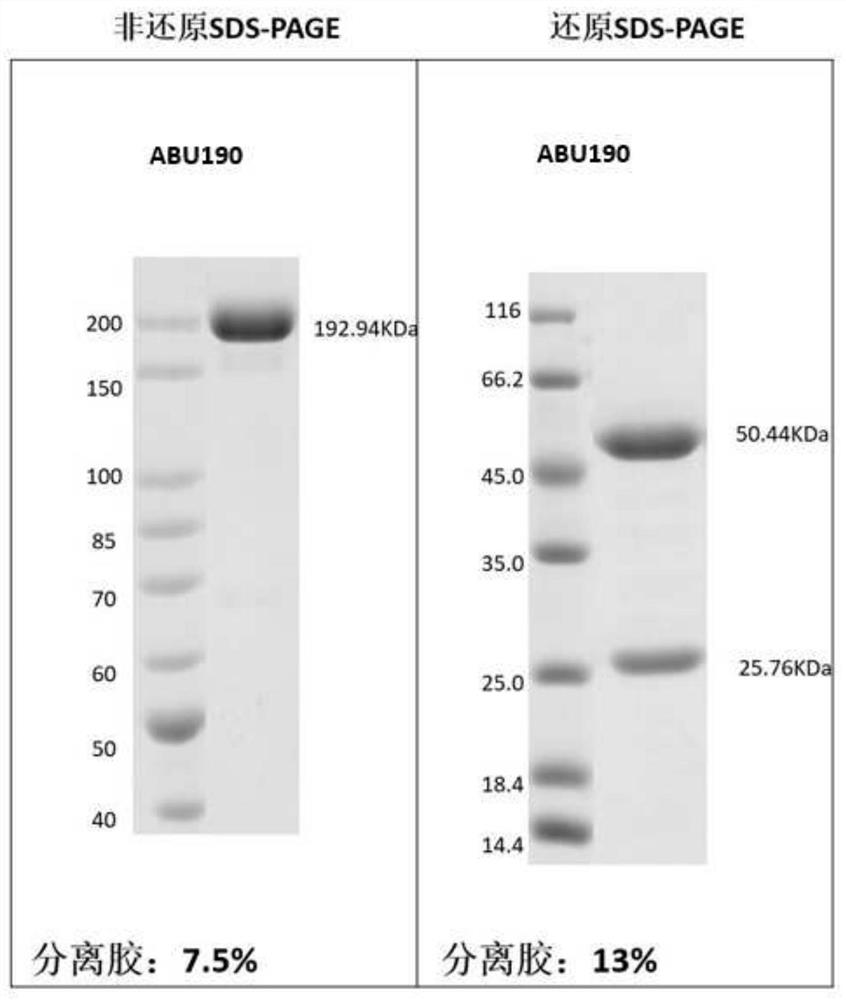
[0254] Example 3: Preparation and purification of antigen binding units herein
[0255] According to the sequence information of the antigen-binding units obtained in Example 2, Beijing Yiqiao Shenzhou Co., Ltd. was commissioned to express and purify the obtained antigen-binding units, and their antigen reactivity was tested.
[0256] Briefly, nucleic acid molecules encoding antibody heavy and light chains were synthesized in vitro, and then cloned into expression vectors, respectively, to obtain recombinant expression vectors encoding antibody heavy and light chains, respectively. HEK293 cells were co-transfected with the above-mentioned recombinant expression vectors encoding antibody heavy chain and light chain respectively. After 4-6 hours of transfection, the cell culture medium was replaced with serum-free medium, and cultured at 37°C for 6 days. After the culture is over, the antibody protein expressed by the cells is purified from the culture through an affinity purif...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
| Antibody titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com