Preparation method of semaglutide dipeptide side chain
A side chain and dimethylformamide technology, which is applied in the field of preparation of semaglutide dipeptide side chains, can solve the problems of difficult product separation, long synthesis period, many synthesis steps, etc., and achieves simplified operation steps and reaction rate. Fast, high-yield effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
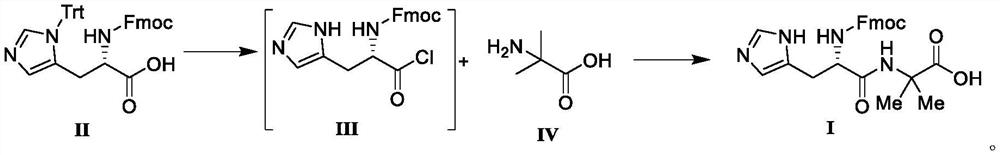
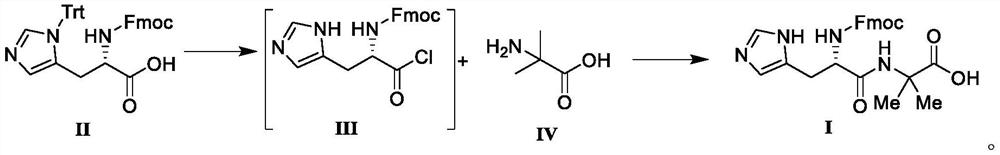
[0031] Add N-protected-L-histidine (6.20g, 0.01mol) in the reaction flask, thionyl chloride (9.52g, 0.08mmol) and heat to reflux, add DMF (30mL), control temperature and reflux, reduce The unisolated intermediate III (according to 100% yield) was distilled under high pressure with solvent.
[0032] Add 2-aminoisobutyric acid (1.24g, 12.0mmol), triethylamine (1.21g, 12.0mmol), dichloromethane (20mL) into the reaction flask, lower to 0°C, add the above compound III (4.32g, 10.0 mmol) was incubated for reaction, and after TLC detected that the reaction was complete, water (20 mL) was added to the reaction system and stirred for 10 minutes, the organic phase was collected, and concentrated under reduced pressure until no liquid flowed out. Add dichloromethane / methyl tert-butyl ether (30mL, V 二氯甲烷 :V 甲基叔丁基醚 =1:1) mixed solution, stirred and crystallized at room temperature, and filtered to obtain a white solid, compound I, with a yield of 98.2% and a purity of 99.87% by HPLC.
Embodiment 2
[0034] N-protected-L-histidine (6.20g, 0.01mol) was added to the reaction flask, thionyl chloride (7.14g, 0.06mmol) was heated to reflux, N,N-dimethylacetamide (30mL) was added, Control the temperature and reflux. After the reaction is completed, the unisolated intermediate III (according to the yield of 100%) is distilled under reduced pressure.
[0035] Add 2-aminoisobutyric acid (1.24g, 12.0mmol), triethylamine (1.21g, 12.0mmol), dichloromethane (20mL) into the reaction flask, lower to 0°C, add the above compound III (4.32g, 10.0 mmol) was incubated for reaction, and after TLC detected that the reaction was complete, water (20 mL) was added to the reaction system and stirred for 10 minutes, the organic phase was collected, and concentrated under reduced pressure until no liquid flowed out. Add dichloromethane / methyl tert-butyl ether (30mL, V 二氯甲烷 :V 甲基叔丁基醚 =1:1) mixed solution, stirred and crystallized at room temperature, and filtered to obtain a white solid, compound I,...
Embodiment 3
[0037] N-protected-L-histidine (6.20g, 0.01mol) was added to the reaction flask, thionyl chloride (11.9g, 0.1mmol) was heated to reflux, N,N-dimethylformamide (30mL) was added, Control the temperature and reflux. After the reaction is completed, the unisolated intermediate III (according to 100% yield) is distilled under reduced pressure.
[0038] Add 2-aminoisobutyric acid (1.24g, 12.0mmol), triethylamine (1.21g, 12.0mmol), chloroform (20mL) into the reaction flask, lower to 0°C, add the above compound III (4.32g, 10.0 mmol) was incubated for reaction, and after TLC detected that the reaction was complete, water (20 mL) was added to the reaction system and stirred for 10 minutes, the organic phase was collected, and concentrated under reduced pressure until no liquid flowed out. Add dichloromethane / methyl tert-butyl ether (30mL, V 二氯甲烷 :V 甲基叔丁基醚 =1:1) mixed solution, stirred and crystallized at room temperature, and filtered to obtain a white solid, compound I, with a yield...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com