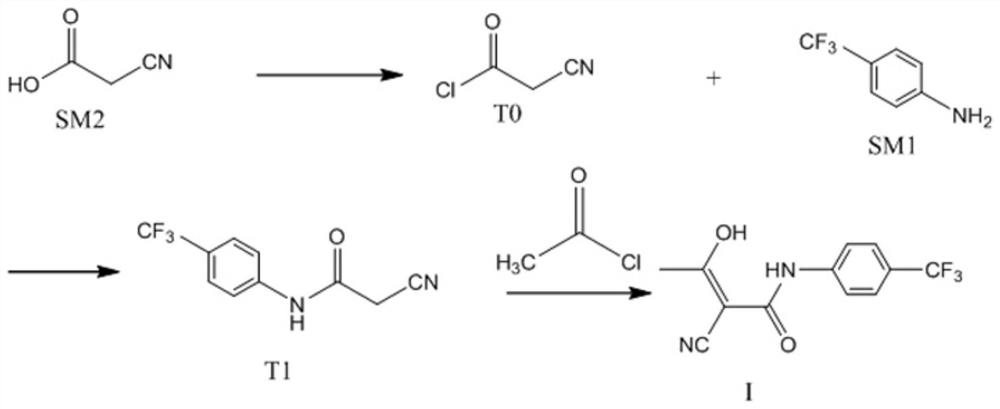
Continuous flow teriflunomide preparation process
A technology for teriflunomide and preparation process, which is applied in the field of continuous flow teriflunomide preparation technology, can solve problems such as hazards, and achieve the effects of solving the impact of ring damage, stable process operation, and reducing potential safety hazards.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] A. Preparation of cyanoacetyl chloride T0
[0043] A1: dissolved 30 g of cyanoacetic acid SM2 in 150 ml of tetrahydrofuran;
[0044] A2: 80.78 g of phosphorus addition in 500 ml of tetrahydrofuran is formulated in a mixed solution;
[0045]a3: the solution from step a1 and step a2 prepared was passed via a metering pump to the microchannel reactor, the flow rate of the solution wherein step a1 prepared was 20g / min, the flow rate of the solution of step a2 prepared was 64g / min; 25 ℃, a reaction under atmospheric pressure 100s, cyano chloride to give T0;
[0046] B. Preparation intermediate-2-cyano-N- [4- (trifluoromethyl) phenyl] -ecetamide T1
[0047] b1: The SM1 38g trifluoromethylaniline was dissolved in 380ml of tetrahydrofuran, was added 76g triethylamine;
[0048] b2: The cyanoacetyl chloride prepared in step b1 T0 and step A was separately conveyed via a metering pump into a microchannel reactor, wherein step b1 solution flow rate is 28g / min, T0 flow rate of 53g...
Embodiment 2
[0054] A. Preparation of cyanoacetyl chloride T0
[0055] a1: 30g of acetic acid was dissolved in 300ml cyano SM2 dichloromethane;
[0056] A2: Preparation chloro reagents: 55g thionyl chloride was added to 500ml of tetrahydrofuran was prepared a mixed solution;
[0057] a3: The solution prepared in step a1 and step a2 are passed via a metering pump into a microchannel reactor, wherein the flow rate of the solution prepared in step a1 of 50g / min, the flow rate of the solution prepared in step a2 of 6.7g / min; 30 ℃, at one atmosphere pressure, the reaction 100s, cyano chloride to give T0;
[0058] B. Preparation intermediate-2-cyano-N- [4- (trifluoromethyl) phenyl] -ecetamide T1
[0059] b1: The SM1 38g trifluoromethylaniline was dissolved in 380ml of dichloromethane, 95g of triethylamine was added;
[0060] b2: The cyanoacetyl chloride prepared in step b1 T0 and step A was separately conveyed via a metering pump into a microchannel reactor, wherein step b1 solution flow rate is...
Embodiment 3
[0066] A. Preparation of cyanoacetyl chloride T0
[0067] a1: 30g of acetic acid was dissolved in 600ml cyano SM2 dichloromethane;
[0068] a2: preparing chlorinated reagents: 68g of phosphorus trichloride was added 680ml of methylene chloride, stirring and blending;
[0069] a3: The solution prepared in step a1 and step a2 are passed via a metering pump into a microchannel reactor, wherein the flow rate of the solution prepared in step a1 of 60g / min, the flow rate of the solution prepared in step a2 is 70.5g / min; 30 ℃, at one atmosphere pressure, the reaction was completed, acetyl chloride to give a cyano T0;
[0070] B. Preparation intermediate-2-cyano-N- [4- (trifluoromethyl) phenyl] -ecetamide T1
[0071] b1: The SM1 38g trifluoromethylaniline was dissolved in 570ml of methylene chloride, 190g of triethylamine were added;
[0072] b2: The cyanoacetyl chloride prepared in step b1 T0 and step A was separately conveyed via a metering pump into a microchannel reactor, wherein ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com