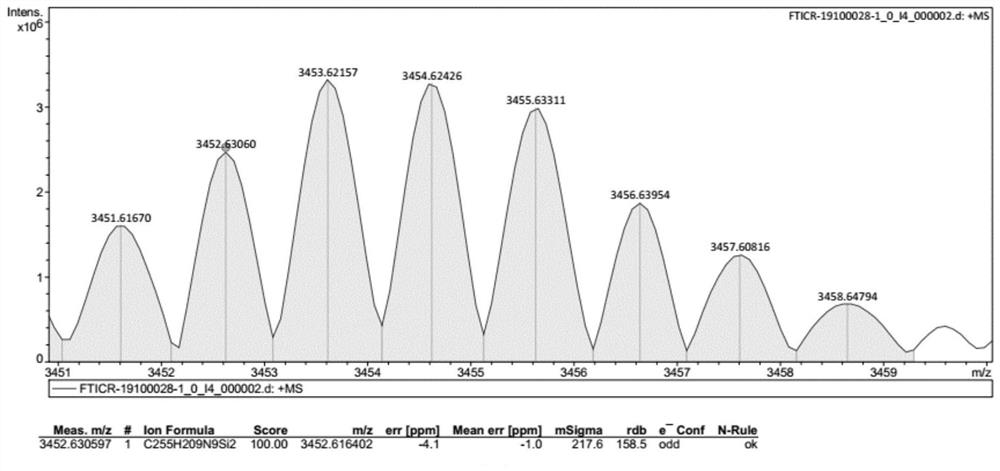
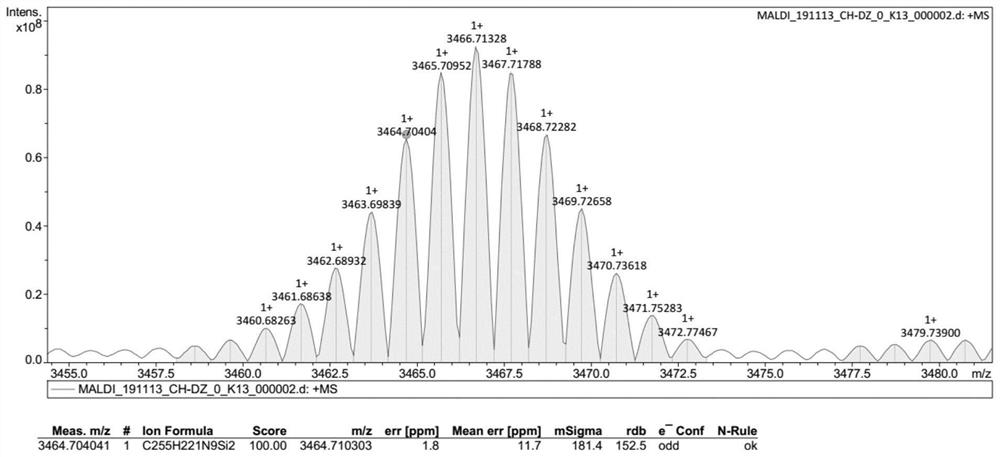
Compound with macrocyclic structure, preparation method of compound and application of compound in synthesis of capsule-shaped molecular cage
A technology of compounds and general formulas, applied in the field of organic synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Preparation of compound 2, the synthetic route is as follows:
[0086]
[0087] Take compound 1 (2.5g, 10mmol) in a 250mL eggplant-shaped bottle preloaded with a magnet, and add solvent tetrahydrofuran (40mL). Place the eggplant-shaped bottle in an ice-water bath and stir at 0°C, weigh NaH (0.8g, 60wt%, 20mmol, 2eq.) into the bottle, and continue stirring at 0°C for 1h. Add 1-bromooctane (3.85g, 20mmol, 2eq.) to the eggplant-shaped flask, and reflux at 77°C for 8h. After the reaction was completed, it was lowered to room temperature, and H 2 O (10 mL) quenched the reaction. The product was extracted with dichloromethane (DCM), then the organic phase was washed with saturated NaCl solution, and washed with anhydrous NaCl 2 SO 4 Remove water from the organic phase. After the solvent was removed by rotary evaporation, the reaction system was separated by column chromatography (eluent: petroleum ether (PE)) to obtain compound 2 with a yield of 96%.
[0088] 1 H NM...
Embodiment 2
[0090] Preparation of compound 5, the synthetic route is as follows:
[0091]
[0092] Get compound 2 (1.8g, 5mmol) in the 250mL eggplant-shaped bottle of prepacked magnetic son, in N 2 THF (30 mL) was added into the environmental glove box to dissolve compound 2, and the reaction vial was sealed and transferred out. The eggplant-shaped flask was pre-stirred in a -78°C cold bath for 15min, n-butyllithium reagent (1.6M, 3mL, 5mmol, 1eq.) was added dropwise, and the stirring was continued at -78°C for 1.5h. Compound 4 (1.69g, 5mmol, 1eq.) was dissolved in THF (20mL), an anhydrous and oxygen-free solvent, and transferred into an eggplant-shaped flask at -78°C. After the transfer was complete, the cooling bath was warmed to -60°C and stirred for 1 hour. The reaction flask was taken out, and stirring was continued for 8 h at room temperature (25° C.). After the reaction is complete, add H 2 The reaction was quenched with O (10 mL), and the product was extracted with DCM and ...
Embodiment 3
[0095] Preparation of compound 6, the synthetic route is as follows:
[0096]
[0097] Get compound 5 (500mg, 0.8mmol) in the 250mL eggplant-shaped bottle of prepacked magnetic son, in N 2 The substrate was dissolved in toluene (50 mL) in an environmental glove box, and the vial was sealed and transferred out. Methanesulfonic acid (5 μL) was added to the reaction vial using a microsampler to obtain a dark purple solution, which was continuously stirred at 80° C. for 4 h. After the reaction, the color of the reaction system faded, and after cooling down to room temperature, triethylamine (5 mL) was added to quench the reaction. The solvent was removed by a rotary evaporator, and the reaction system was separated and purified by column chromatography (eluent PE:DCM=5:1) to obtain 154 mg of macrocyclic compound 6.
[0098] 1 H NMR (400MHz, CDCl 3 )δ7.59(d, J=8.0Hz, 6H), 7.55(s, 6H), 7.49(d, J=8.4Hz, 6H), 7.07(d, J=8.4Hz, 12H), 6.84(d, J=8.0Hz, 12H), 3.51(t, J=7.6Hz, 6H), ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com