Method for determining etomidate emulsion injection in-vitro release curve
A technology for etomidate and a determination method is applied in the field of determination of the external release curve of etomidate emulsion injection, which can solve the problem of not establishing a determination method for etomidate emulsion injection, and achieves reduction of research and development risks, reliable quality control, and high reliability. The effect of improving the test pass rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
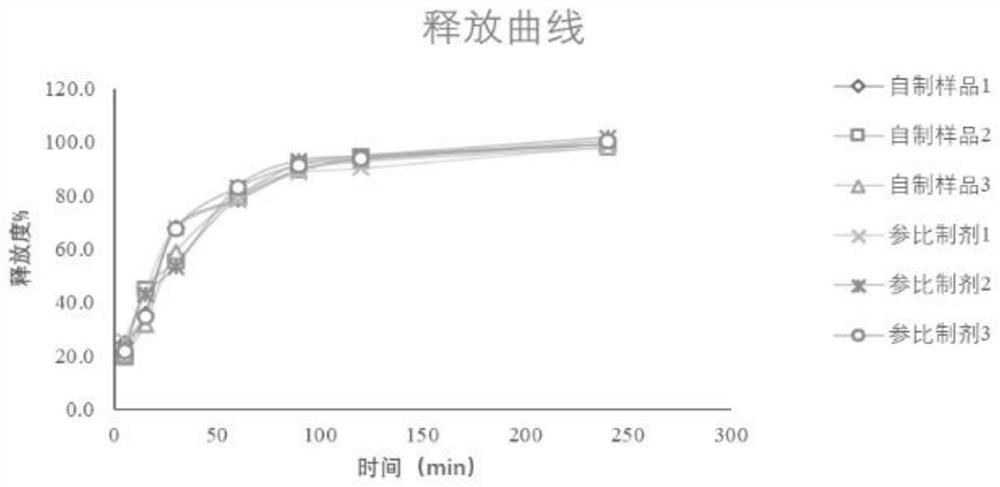
[0047] Embodiment 1: Adopt basket method-dynamic dialysis method
[0048] Using the device in the four appendices of the Chinese Pharmacopoeia 2020 edition, prepare 500 mL of phosphate buffer saline PBS containing 30% absolute ethanol at pH 7.4 as the release medium at a speed of 100 rpm / min and a temperature of 37°C±0.5°C. Put the dialysis bag (molecular weight cut-off 8kD-15kD) containing 5mL of etomidate emulsion injection into the rotating basket, immerse in the release medium, absorb 1mL of the drug release solution at each sampling point, and supplement the isothermal release medium at the same time.
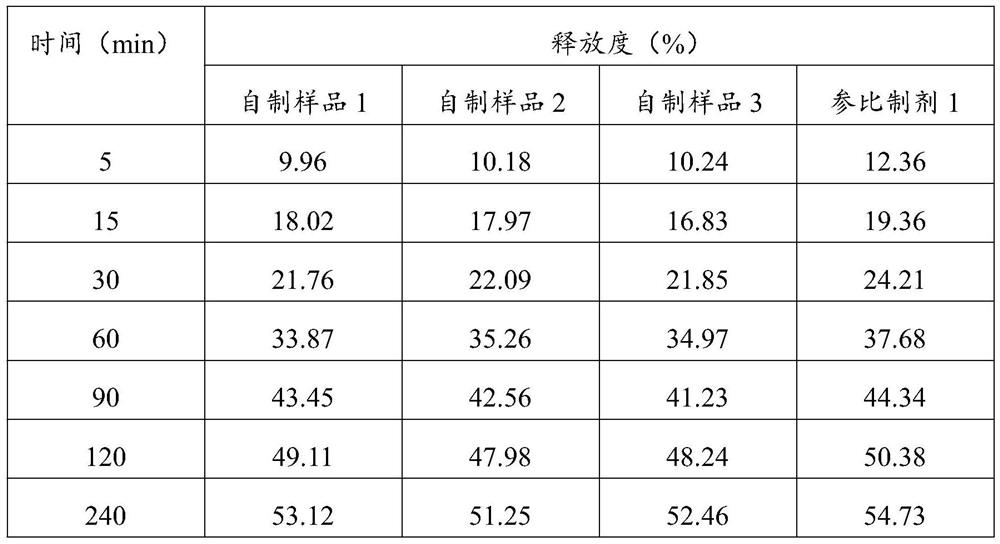
[0049]Utilize above-mentioned method, assay batch number is the etomidate emulsion injection of self-made sample 1, self-made sample 2, self-made sample 3 and reference preparation 1, and the results are shown in Table 1 below.
[0050] Table 1 basket method-dynamic dialysis method release test results
[0051]
[0052] The results in Table 1 show that using the method...
Embodiment 2
[0053] Embodiment 2: adopt paddle method-reverse dialysis method
[0054] Using the dissolution apparatus of the four appendices of the Chinese Pharmacopoeia 2020 edition, prepare 500mL of phosphate buffer saline PBS containing 30% absolute ethanol at pH 7.4 as the release medium, the speed is 100r / min, and the temperature is 37°C±0.5°C. The dialysis bag (molecular weight cut-off 8kD-15kD) filled with 2mL release medium was immersed in the release medium for 2h to equilibrate. Precisely measure 5 mL of etomidate emulsion injection and directly add it to the release medium outside the dialysis bag, take 0.5 mL of the release medium outside the dialysis bag at 0 min as the total drug solution, add 0.5 mL of tetrahydrofuran, mix and filter. Take out the dialysis bag at each sampling point, pour out the solution in the bag, take 0.5 mL of the solution as the drug release solution, add 0.5 mL of tetrahydrofuran, mix to 1 mL, and filter.
[0055] Utilize above-mentioned method, ass...
Embodiment 3
[0061] Embodiment 3: adopt flow cell method-dynamic dialysis method
[0062] Adopt the United States Pharmacopoeia dissolution test flow cell closed system device, 22.6mm flow cell, prepare 200mL of phosphate buffer saline PBS containing 30% absolute ethanol at pH 7.4, as the release medium, the flow rate is 8mL / min, and the temperature is 36 ~37.7°C. A dialysis bag (molecular weight cut-off 8kD-15kD, diameter 8mm) containing 2mL of etomidate emulsion injection was placed in a cuvette, and 1mL of the drug release solution was drawn at each sampling point, and an equal amount of isothermal release medium was added at the same time.
[0063] Utilize above-mentioned method, assay batch number is the etomidate emulsion injection of self-made sample 1, self-made sample 2, self-made sample 3 and reference preparation 1, and the results are shown in Table 3 below.
[0064] Table 3 flow cell method-dynamic dialysis method release test results
[0065]
[0066] The results in Tabl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com