Composition, for preventing, relieving or treating cartilage-related diseases or symptoms, comprising hapln1
A composition, a technology of cartilage tissue, applied in the field of compositions for diseases or symptoms, compositions for improving or treating dwarfism, compositions for improving or treating osteoarthritis, prevention, and compositions for regenerating cartilage, capable of solving problems without Specific mention of prevention, improvement or treatment, etc., to achieve the effect of small side effects and promoting bone growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1: Analysis of cartilage regeneration ability of HAPLN1 protein in degenerated cartilage tissue in vivo
[0056] 1-1. Promotion of cartilage formation in degenerated growth plate by repeated intraperitoneal administration of HAPLN1 protein
[0057] The 6-week-old male C57BL / 6 mice were classified into the young (Young) group, and the 20-month-old C57BL / 6 mice were classified into the aging (Old) group. In the aging group, the HAPLN1 protein was diluted in phosphoric acid Buffered saline (phosphate buffered saline: PBS) was intraperitoneally administered at a dose of 0.1 mg / kg for 2 weeks, and the control group was intraperitoneally administered PBS in the same manner.
[0058] Take the femur and knee joints of mice in each group, and fix them with neutral buffered 10% formalin (NBF) for 48 hours, and then use 10% ethylenediaminetetraacetic acid (EDTA) sequentially The solution was decalcified for 7 days. Subsequently, each specimen was embedded in paraffin t...
Embodiment 2
[0064] Example 2: Analysis of cartilage regeneration ability of HAPLN1 protein in damaged cartilage tissue in vivo
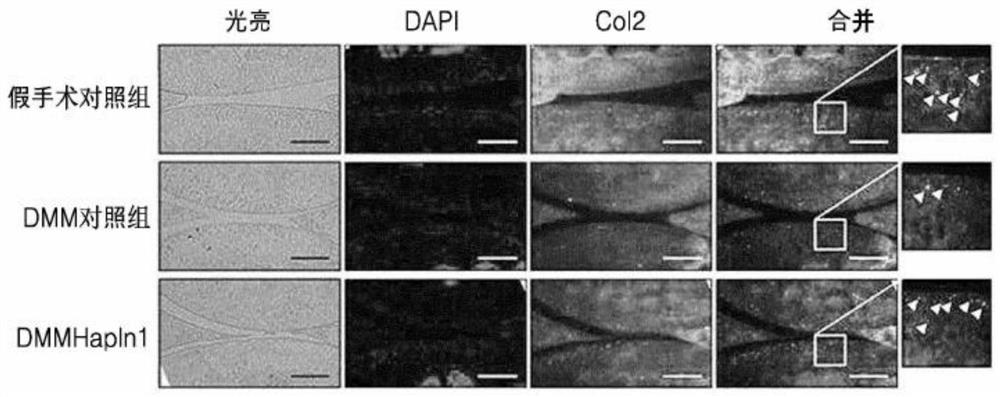
[0065] Seven-week-old male C57BL / 6 mice were divided into the following three groups. The normal control group (sham operation control group) was a simulated operation group (shamoperation) regarding destabilization of medial meniscus (DMM) operation, and they were raised under the existing conditions for 4 weeks after the operation. In the vehicle (Vehicle) treatment group (DMM control group), they were raised under the existing conditions for 8 weeks after the DMM operation, and PBS was intra-articularly administered once a week for the last 4 weeks. In the HAPLN1 treatment group (DMM HAPLN1 group), they were raised under the existing conditions for 8 weeks after the DMM operation, and the HAPLN1 protein was diluted in PBS at a concentration of 1 μg / mL once a week for the last 4 weeks and joints were performed. Administered internally.
[0066] At the end of...
Embodiment 3
[0069] Example 3: Analysis of the ability of HAPLN1 protein to promote cartilage formation in vitro
[0070] 3-1. HAPLN1 protein increases the cartilage formation ability of human articular chondrocytes
[0071] Human articular chondrocytes (human articular chondrocyte: HAC) contained 10% fetal bovine serum (fetal bovine serum; FBS; Gibco), 1% penicillin / streptomycin (Gibco) and 1% non-essential Amino acid (non-essential amino acid; NEAA; Gibco) DMEM (Dulbecco's modified Eagle medium) medium and F12 mixed in a 1:1 (DMEM / F12; Gibco) medium at 37 ° C and 5% CO 2 cultured under conditions.
[0072] As a model for testing the chondrogenic ability of HAC, a three-dimensional culture system in which cells were embedded in alginate beads was used. The HAC was uniformly mixed in a 1.25% alginate solution, and each bead contained 30,000 cells. They were prepared by adding 50 μg / mL L-ascorbic acid 2-phosphate, 1% insulin-transferrin-selenium (ITS; Gibco) and 10 ng / mL TGF-β1 to the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com