Water-soluble mitochondria-targeted silicon phthalocyanine axially substituted by ethyoxyl bromopropyl imidazole and preparation method and application thereof
A technology of bromopropyl and silicon phthalocyanine, applied in the field of complexes, to achieve the effect of improving the interaction between positively charged phthalocyanine and negatively charged mitochondrial membranes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
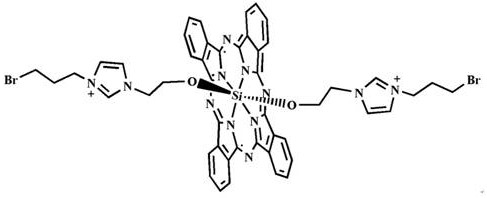
[0019] 1) Dichlorosilicon phthalocyanine (SiPcCl 2 )Synthesis
[0020] Add 1,3-diiminoisoindoline (7.28 g, 50.15 mmol), silicon tetrachloride (8.30 mL) and quinoline (83 mL) into a 250 mL three-necked flask, and stir at 220 °C Reflux for 1 h, when the reaction solution drops to 70°C, pour it into 500 mL of methanol solution, stir and let stand, then filter, wash the precipitate with acetone, methanol, dichloromethane and other solvents, and vacuum dry to obtain 3.68 g of purple-red solid , and the yield was 48.62%.
[0021] ) Synthesis of 1-(2-hydroxyethyl)-3-(3-bromopropyl) imidazole (abbreviated as Br-ID in the present invention)
[0022] Add 1-(2-hydroxyethyl)imidazole (0.09 g, 0.80 mmol) and 1,3-dibromopropane (0.16 g, 0.80 mmol) into a 100 mL three-necked flask, then add 30 mL of acetonitrile to dissolve them, N 2 Stir and reflux at 80°C for 24 h under protection. After the reaction was completed, it was left to cool for 8 h, and a white solid was precipitated. The so...
Embodiment 1
[0026] In Example 1, in process 2), 1-(2-hydroxyethyl)imidazole was changed to 0.18 g, 1,3-dibromopropane was changed to 0.32 g, other reaction conditions were the same, and the yield was 41.50%.
[0027] In process 3), 1-(2-hydroxyethyl)-3-(3-bromopropyl) imidazole was changed to 0.60 g, dichlorosilicon (IV) phthalocyanine was changed to 0.40 g, other reaction conditions were the same, and the yield is 35.00%.
specific Embodiment 3
[0029] In Example 1, in process 2), 1-(2-hydroxyethyl)imidazole was changed to 0.18 g, 1,3-dibromopropane was changed to 0.48 g, and the yield was 48.92%.
[0030] In process 3), 1-(2-hydroxyethyl)-3-(3-bromopropyl)imidazole was changed to 0.60 g, dichlorosilicon(IV) phthalocyanine was changed to 0.40 g, and the reaction temperature was changed to 150°C. Other reaction conditions are the same, and the yield is 33.85%.
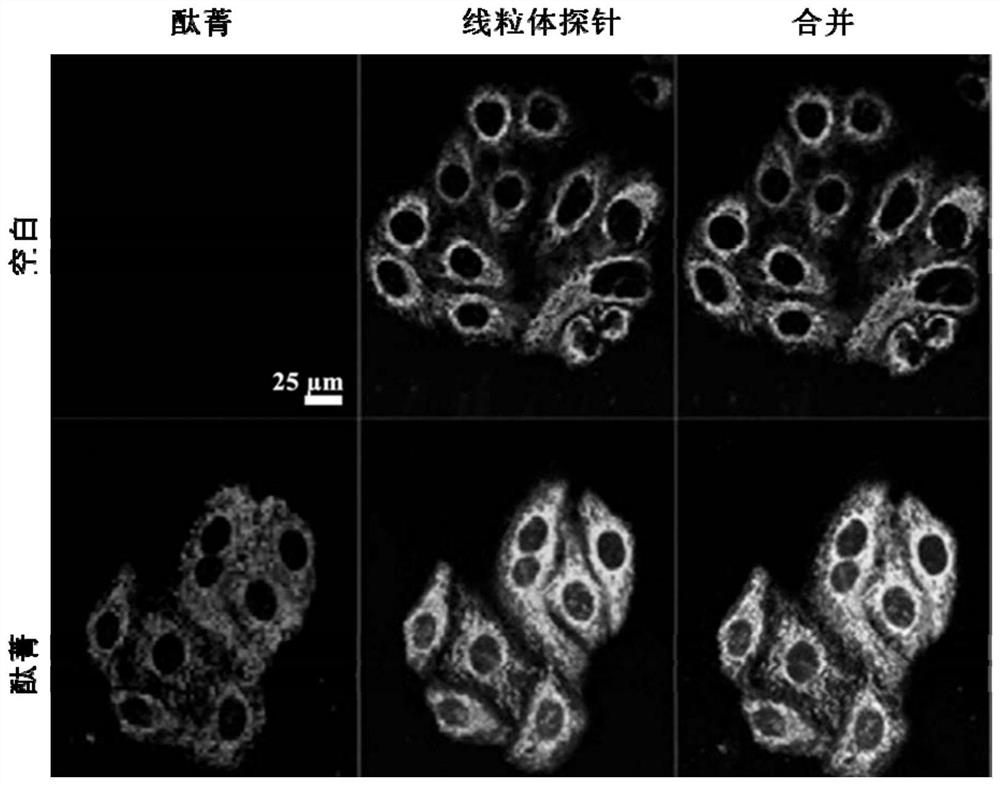
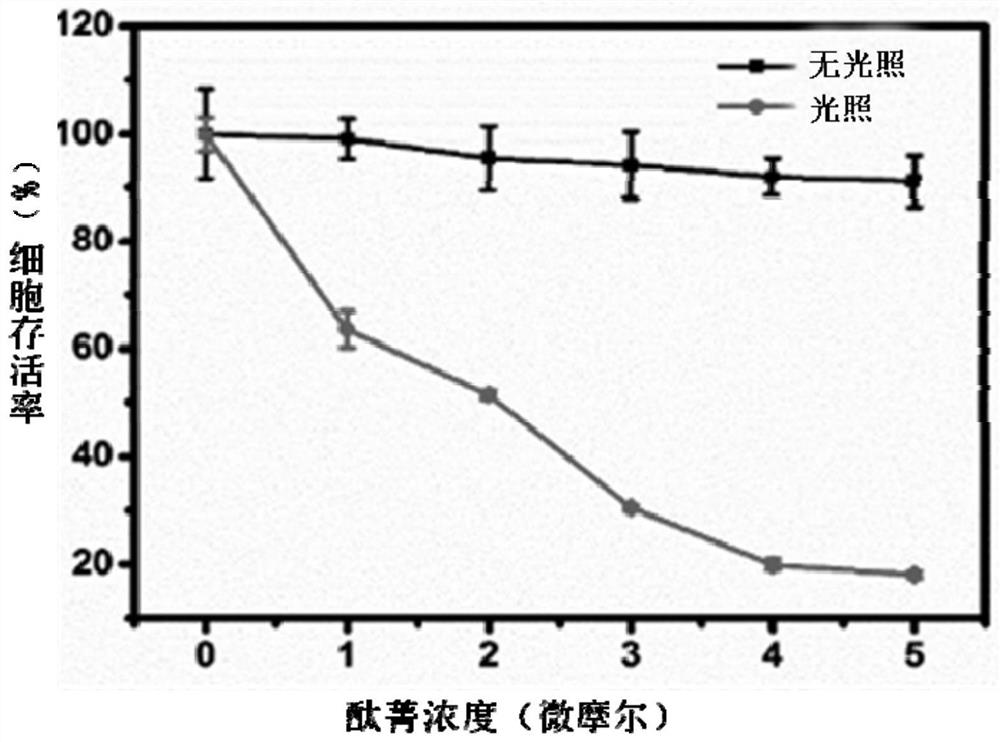
[0031] Mitochondrial-targeted bis-(1-(2-ethoxy)-3-(3-bromopropyl)imidazole) axially substituted silicon phthalocyanine (Br-ID-SiPc) in ovarian cancer cell fluorescence imaging and mitochondria position
[0032] HO-8910 ovarian cancer cells in logarithmic growth phase were digested and centrifuged, resuspended in Dulbecco's Modified Eagle Medium (DMEM) cell culture medium and re-counted, in 1×10 4 The number of cells per well (volume 200 μL) was inoculated in a 20 mm confocal dish, and incubated in a 37 °C constant temperature incubator for 4 h. After the cell...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com