Narrow-band gap non-condensed ring small molecule receptor and preparation method and application thereof
A small-molecule acceptor and narrow-bandgap technology, which is applied in semiconductor/solid-state device manufacturing, electrical solid-state devices, semiconductor devices, etc., can solve the problems of multi-synthesis and purification steps, unfavorable large-scale commercial application of organic photovoltaics, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036]
[0037] (1) Preparation of compound shown in formula IV:
[0038] Under the protection of nitrogen, add the compound shown in formula I (0.70 g, 0.80 mmol) and the compound shown in formula II (2.33 g, 4.00 mmol) and solvent ultra-dry toluene ( 50 mL), after the dissolution was complete, tetrakis(triphenylphosphine)palladium (0.46g, 0.04mmol) was added, heated to 110°C and stirred under reflux for 24h, then cooled to room temperature.
[0039] Post-treatment: Pour the cooled reaction solution into water (100mL) and extract with dichloromethane (100mL×3), wash the organic phase twice with anhydrous MgSO 4 After drying and filtering, the filtrate was removed from the solvent by a rotary evaporator to obtain a crude product. The crude product was separated and purified through a silica gel chromatography column (the effective length of the silica gel was 30cm) using dichloromethane / petroleum ether (1:4 by volume) as the eluent to obtain an intermediate product as a ye...
Embodiment 2
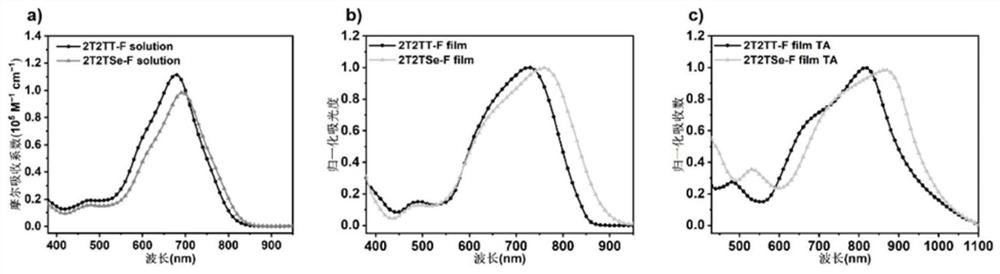
[0058] Embodiment 2 ultraviolet-visible absorption spectrum
[0059] The absorption spectra of the compounds 2T2TT-F and 2T2TSe-F in Example 1 in chloroform solution (10 μM) and in the film state before and after heat treatment were characterized by ultraviolet-visible spectrometer. The former solution was used to measure the UV absorption of the solution, and the latter solution was spin-coated on a quartz plate at 1200 rpm to obtain a thin film, and then the different UV absorptions of the film before and after heat treatment were measured under the conditions of no treatment and heating at 100°C for 5 minutes. , the scanning range is 300nm to 1100nm, the measuring instrument is Jasco V-570UV / VIS / NIR spectrometer, the ultraviolet-visible absorption spectrum is as attached figure 2 shown. The UV-Vis absorption curves of compounds 2T2TT-F and 2T2TSe-F in chloroform are as attached figure 2 As shown in a, the maximum absorption peak wavelengths are 679nm and 694nm respectiv...
Embodiment 3
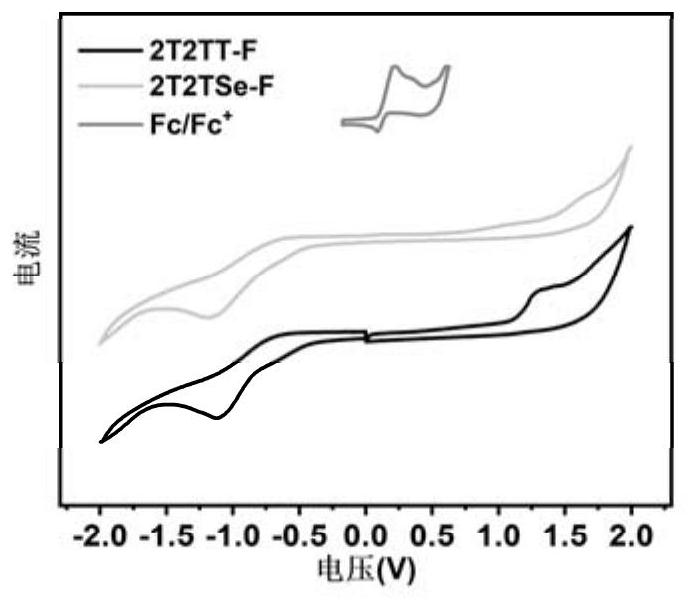
[0060] Embodiment 3 electrochemical analysis
[0061] The electrochemical properties of the compound are tested by the drop film method (frontier orbital energy level and band gap), and the electrolytic cell uses a three-electrode system (glassy carbon electrode as the working electrode, platinum wire electrode as the auxiliary electrode, saturated Calomel electrode as a reference electrode), and the cyclic voltammetry characteristic curve of the compound was tested using an electrochemical workstation. With ferrocene (Ferrocene, Fc / Fc + ) as internal standard, with methanol as solvent, 0.1M tetrabutylammonium hexafluorophosphate (n-Bu 4 NPF 6 ) as the supporting electrolyte, at 100mV s -1 The electrochemical properties of the compounds 2T2TT-F and 2T2TSe-F were tested at the scanning speed, and the cyclic voltammetry characteristic curves are shown in the attached image 3 shown. The calculation formula of electrochemical energy level is as follows: E HOMO =-e(E ,onse...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com