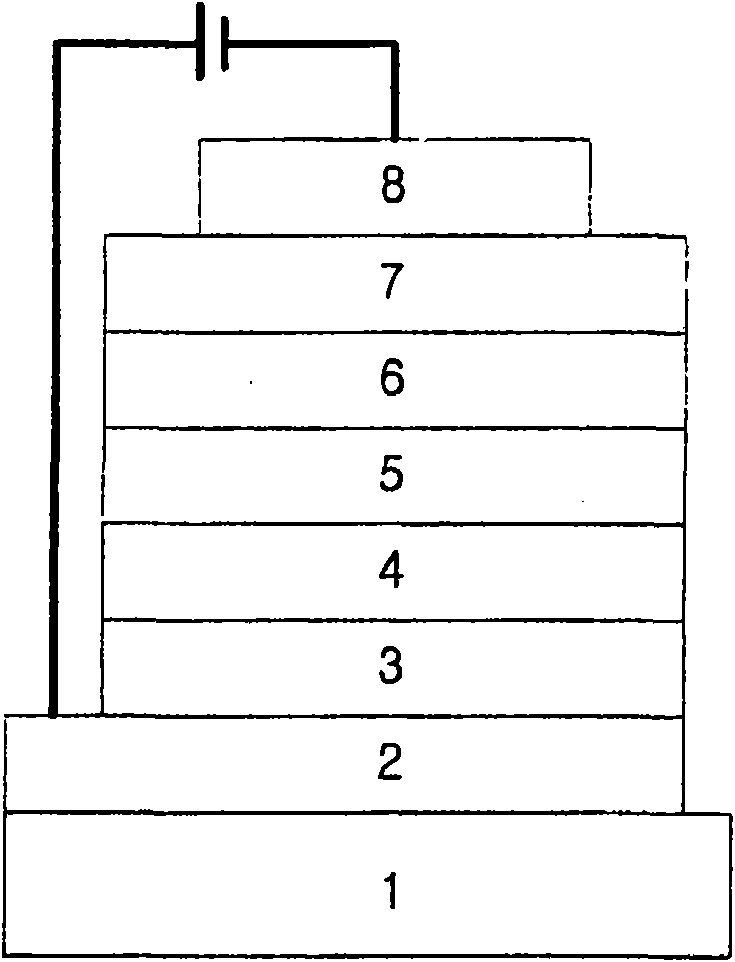
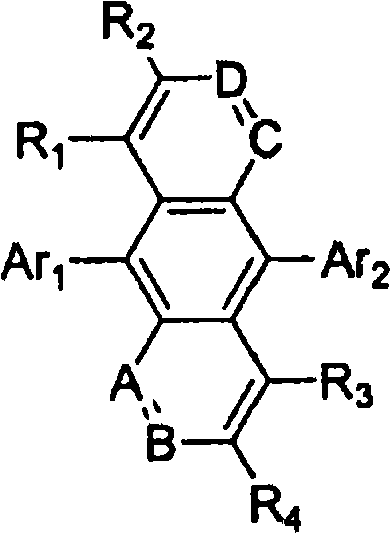
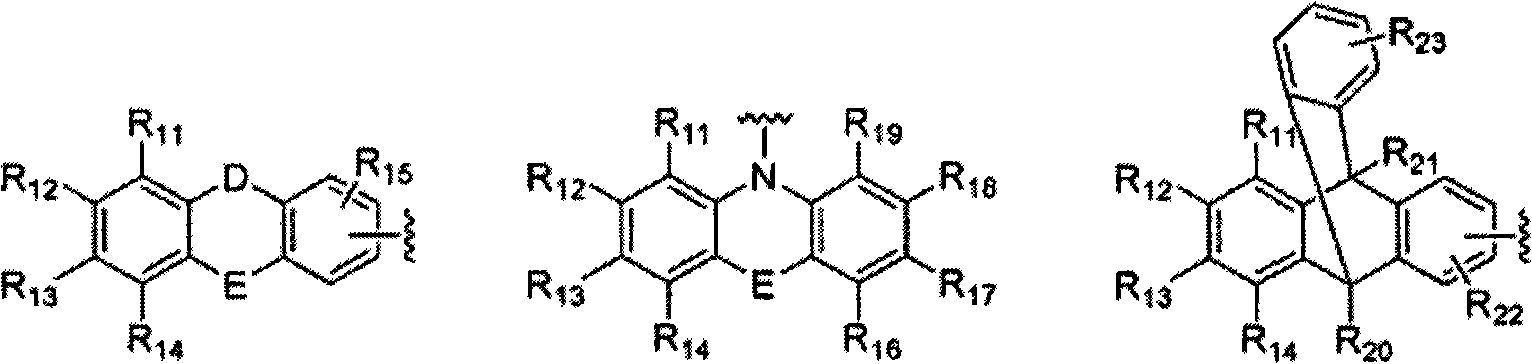
Novel organic electroluminescent compounds and organic electroluminescent device using the same
一种化合物、致发光的技术,应用在有机化学、周期表第4/14族元素的化合物、发光材料等方向,能够解决不能提供纯蓝色光、不适全色显示器等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0191] [Preparation Example 1] Preparation of Compound (8)
[0192]
[0193] Preparation of compound (A)
[0194] Under nitrogen atmosphere, 3-bromopyridine (96 μL, 1 mmol) and diethyl ether (10 mL) were injected into a 50 mL round bottom flask, and the mixture was stirred. After the mixture was cooled to -78°C, butyllithium (2.5 mL, 1 mmol, 2.5M in hexane) was slowly added thereto. After stirring at -78°C for 1 hour, dimethyl phthalate (0.17 mL, 1 mmol) was added slowly at the same temperature. After stirring at -78°C for 2 hours, it was slowly warmed to room temperature, and water (5 mL) was added thereto to effect hydrolysis. The organic layers obtained by extraction with ether were collected and dried. After removal of the solvent, the residue was purified by column chromatography to obtain Compound (A) (0.14 g, 56%) as a solid product.
[0195] Preparation of compound (B)
[0196] Under a nitrogen atmosphere, compound (A) (0.11 g, 0.44 mmol) and THF (5 mL) wer...
preparation Embodiment 2
[0201] [Preparation Example 2] Preparation of compound (383)
[0202]
[0203] Preparation of compound (D)
[0204] Under nitrogen atmosphere, 2-bromopyridine (96 μL, 1 mmol) and diethyl ether (10 mL) were injected into a 50 mL round bottom flask, and the mixture was stirred. After cooling it to -78°C, butyllithium (2.5 mL, 1 mmol, 2.5M in hexane) was slowly added thereto. After stirring at -78°C for 1 hour, dimethyl phthalate (0.17 mL, 1 mmol) was added slowly at the same temperature. After stirring at -78°C for 2 hours, the temperature was slowly raised to room temperature, and water (5 mL) was added thereto to perform hydrolysis. The enriched organic layer obtained by ether extraction was dried. After removal of the solvent, the residue was purified by column chromatography to obtain Compound (D) (0.14 g, 56%) as a solid product.
[0205] Preparation of compound (E)
[0206] Under nitrogen atmosphere, Compound (D) (0.11 g, 0.44 mmol) and THF (5 mL) were poured i...
preparation Embodiment 3
[0211] [Preparation Example 3] Preparation of Compound (758)
[0212]
[0213] Preparation of compound (G)
[0214] Under nitrogen atmosphere, 3-bromopyridine (96 μL, 1 mmol) and diethyl ether (10 mL) were injected into a 50 mL round bottom flask, and the mixture was stirred. After the mixture was cooled to -78°C, butyllithium (2.5 mL, 1 mmol, 2.5M in hexane) was slowly added thereto. After stirring at -78°C for 1 hour, dimethyl phthalate (0.17 mL, 1 mmol) was added slowly at the same temperature. After stirring at -78°C for 2 hours, the temperature was slowly raised to room temperature, and water (5 mL) was added thereto to carry out hydrolysis. The enriched organic layer obtained by extraction with ether was dried. After removing the solvent, the residue was purified by column chromatography to obtain Compound (G) (0.14 g, 56%) as a solid product.
[0215] Preparation of compound (H)
[0216] Under a nitrogen atmosphere, compound (G) (0.11 g, 0.44 mmol) and THF (5 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| fluorescence quantum yield | aaaaa | aaaaa |
| fluorescence quantum yield | aaaaa | aaaaa |
| UV absorption wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com