Anti-RNA (Ribonucleic Acid) virus medicine and application thereof
A RNA virus and drug technology, applied in the field of treatment of viral infections, can solve the problems of long time, high research and development costs, lack of predictability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
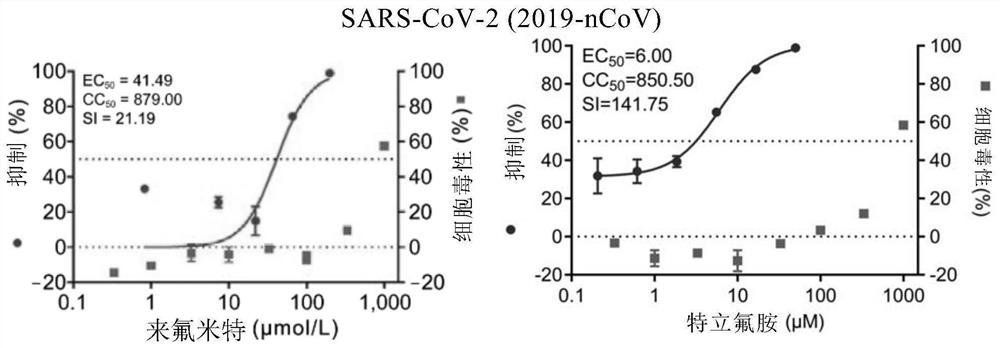
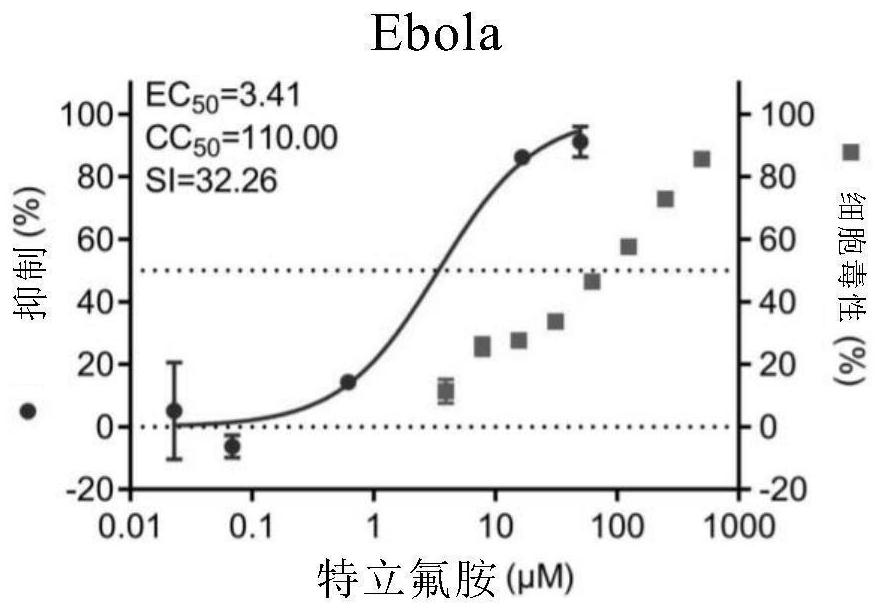
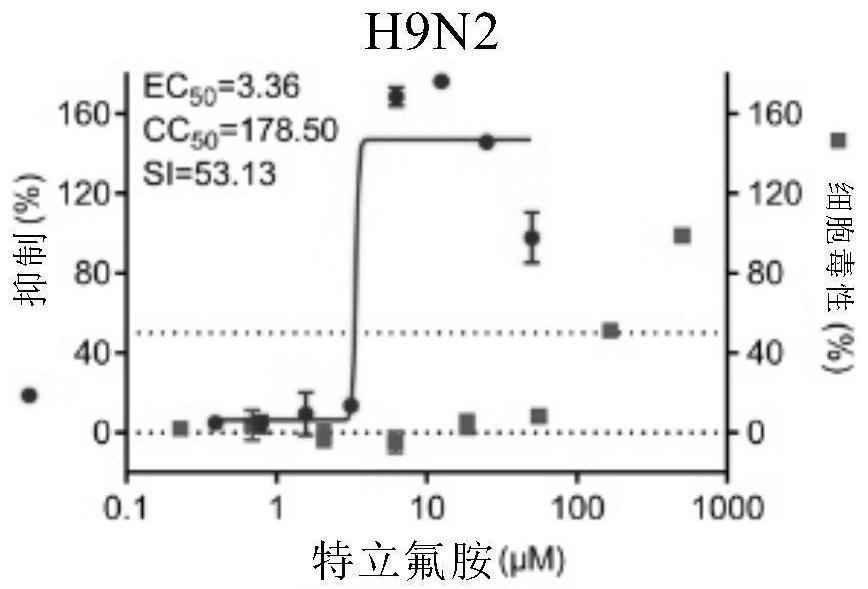
[0112] Embodiment 1. The inhibitory activity and cytotoxicity evaluation of the compound of the present invention to 2019 new coronavirus (2019-nCoV), Ebola virus, avian influenza virus A / GuangZhou / 99 (H9N2)
[0113] Materials and Methods:
[0114] Both leflunomide and teriflunomide are commercially available with a purity of over 98%.
[0115] Detection methods and results:
[0116] 1. Fluorescent quantitative PCR method to detect the efficacy experiment of anti-2019 novel coronavirus:
[0117] On Vero E6 cells (ATCC-1586) with 2019BetaCoV / Wuhan / WIV04 / 2019 strain (isolated by Wuhan Institute of Virology, Chinese Academy of Sciences, Zhou, P., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020) infected with an infection amount of MOI=0.03 or MOI=0.05, at the same time adding different dilutions of the drug for co-culture, the drug was diluted with DMSO, and the DMSO dilution was used as a control. The infection solution was DMEM...
Embodiment 2
[0136] Embodiment 2. The antiviral activity evaluation of the combination of the compound of the present invention and other antiviral drugs
[0137] The inventors further tested leflunomide, teriflunomide and other antiviral drugs of the prior art, including lopinavir, ritonavir, ribavirin, remdesivir, oseltamivir , Tamiflu, laninamivir, peramivir and chloroquine (chloroquine phosphate) in one or more combinations.
[0138] It was found that the combination of leflunomide and teriflunomide with these antiviral drugs can produce better therapeutic effect; (Chloroquine phosphate) combined treatment effect is relatively better.
Embodiment 3
[0139] Example 3. Drug efficacy evaluation of leflunomide against influenza virus in mouse infection model
[0140] Experimental Materials:
[0141] Leflunomide is commercially available with a purity of over 98%.
[0142] Detection method:
[0143] In this experiment, the anti-influenza efficacy of the drug was evaluated based on the mouse influenza A / WSN / 33 (H1N1) infection model. The mice used were BALB / c female mice, 6-8 weeks old, weighing 18-22g, purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd., and all animal experiments were performed in the ABSL-2 laboratory. After the mice adapted to the ABSL-2 environment for 2-3 days, the experiment was carried out, and the mice were divided into the following 4 groups: model group: Non-treatment; positive control group: Osel (oseltamivir) 20mg / kg; experimental group: Lef ( Leflunomide) 20mg / kg and 10mg / kg; 3 to 6 rats in each group. With A / WSN / 33(H1N1)2LD 50 =2000pfu / toxic dose carried out nasal d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com