Sacubitril and valsartan sodium single-layer osmotic pump controlled release tablet and preparation method thereof
A technology of sacubitrilvaler and single-layer osmotic pump, which is applied in coatings, pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve the problems of slow release rate and susceptibility to external environment, and reduce the Toxic and side effects, the effect of reducing the total dose of medication and reducing the frequency of medication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
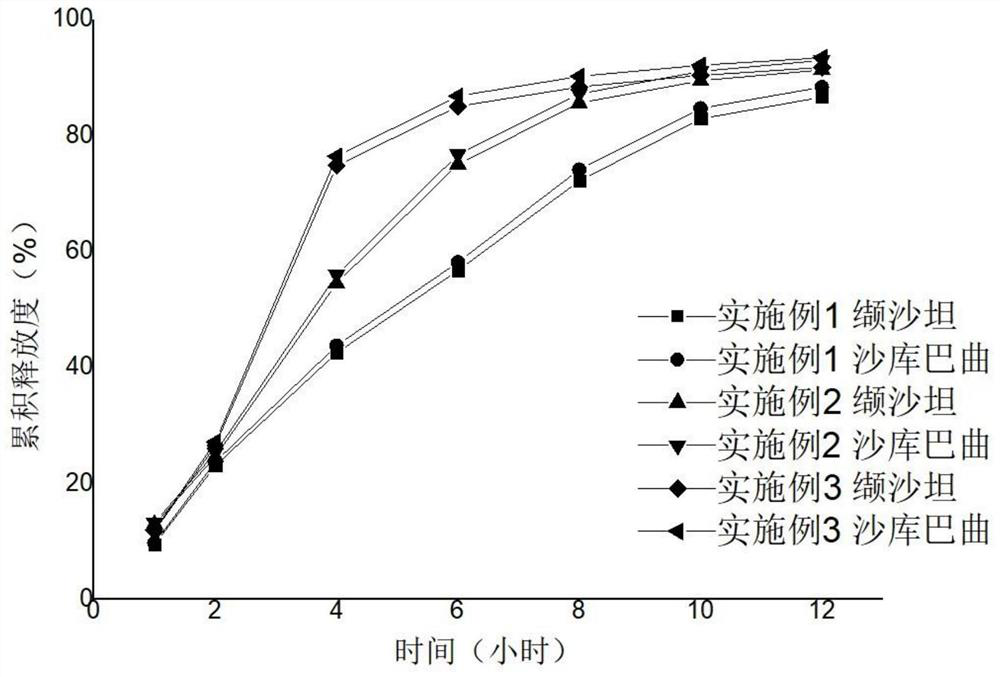
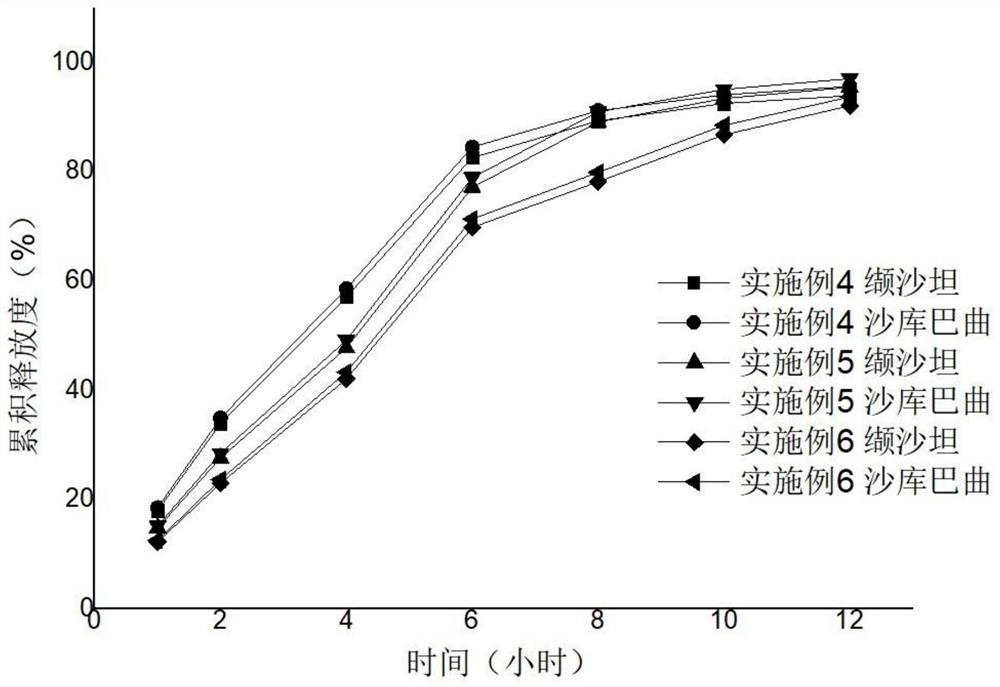
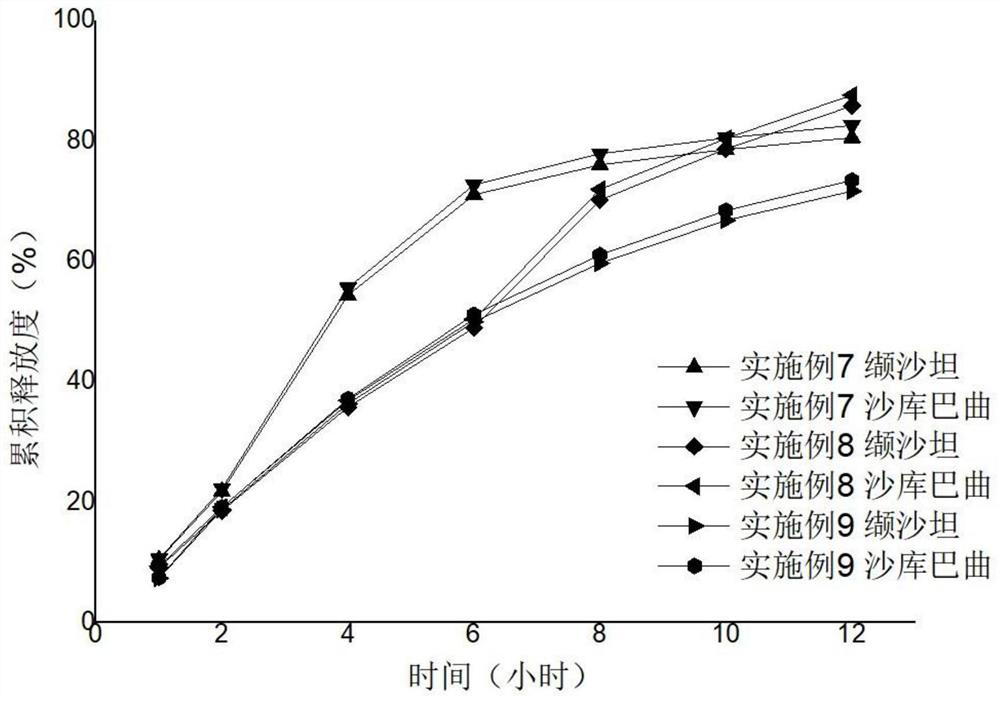
[0034]Examples 1 to Example 15 Preparation method: Weighing a variety of drugs and excipients (except for magnesium stearate), mix uniformly in an equal amount. Add appropriate amount of wetting agent anhydrous ethanol soft material, over 20 mesh sieves, 40 ° C for 0.5 hours, and 20 mesh sieve dry. Magnesium stearate is mixed. A circular shallow dimming die or 14 * 9 mm diamond shallow dimming die or 18.4 * 7.2mm, a shallow concave mold, and a hardness of 6-8 kg. According to the calculation of cellulose and polyethylene glycol 3350, 35 ° C, the heating water bath is added, and the magnetic stirring is added to the acetone - aqueous solution (94.525: 0.75), and the coating liquid is clear and transparent. The core is placed in the coating pot, and the temperature of the bed temperature is 22 to 29 ° C, and the coating is 3 to 10%. After the coating was completed, the aging was placed in an oven at 40 ° C for 12 hours to remove acetone. An interpretation hole having a diameter of 0...
Embodiment 1
[0037] Core prescription
[0038] composition Dosage (per piece) Sandaki vals 452mg Polyethylene pyrrolidone K90F 36mg Lactose G200 40mg Magnesium stearate 12mg
[0039] Coated film prescription
[0040] composition Dosage (per 100 pieces) Cellulose acetate 1.87g Polyethylene glycol 3350 0.47g acetone 44.24g water 0.22g
Embodiment 2
[0042] Core prescription
[0043] composition Dosage (per piece) Sandaki vals 452mg Polyethylene pyrrolidone K90F 24mg Lactose G200 52mg Magnesium stearate 12mg
[0044] Coated film prescription
[0045] composition Dosage (per 100 pieces) Cellulose acetate 2.71g Polyethylene glycol 3350 0.68g acetone 64.09g water 0.32g
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com