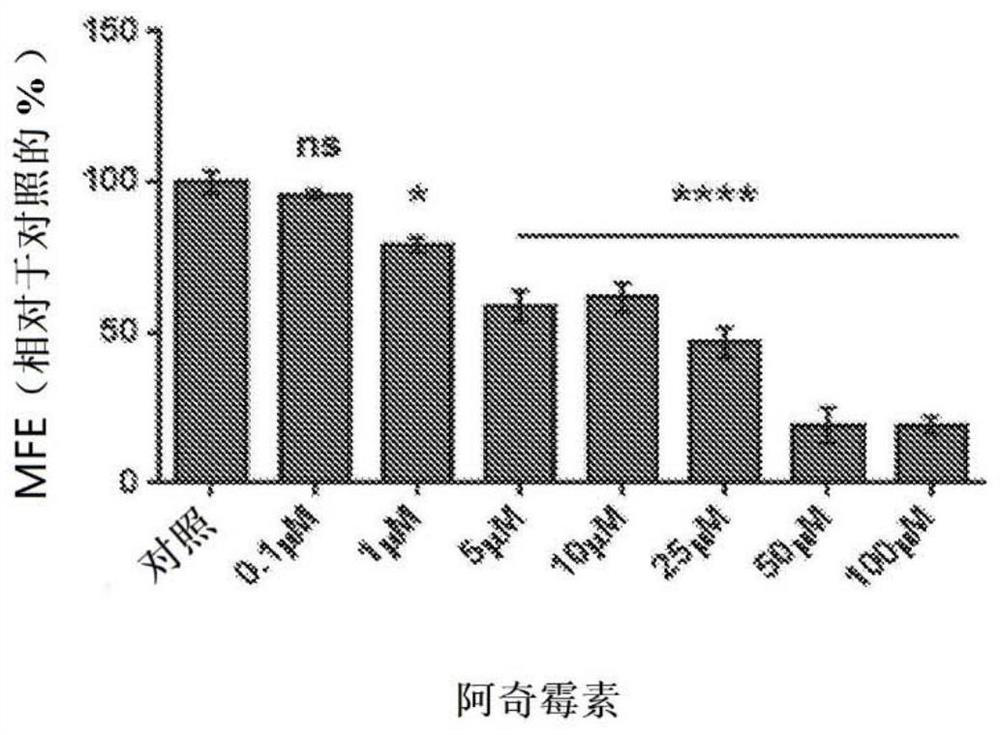
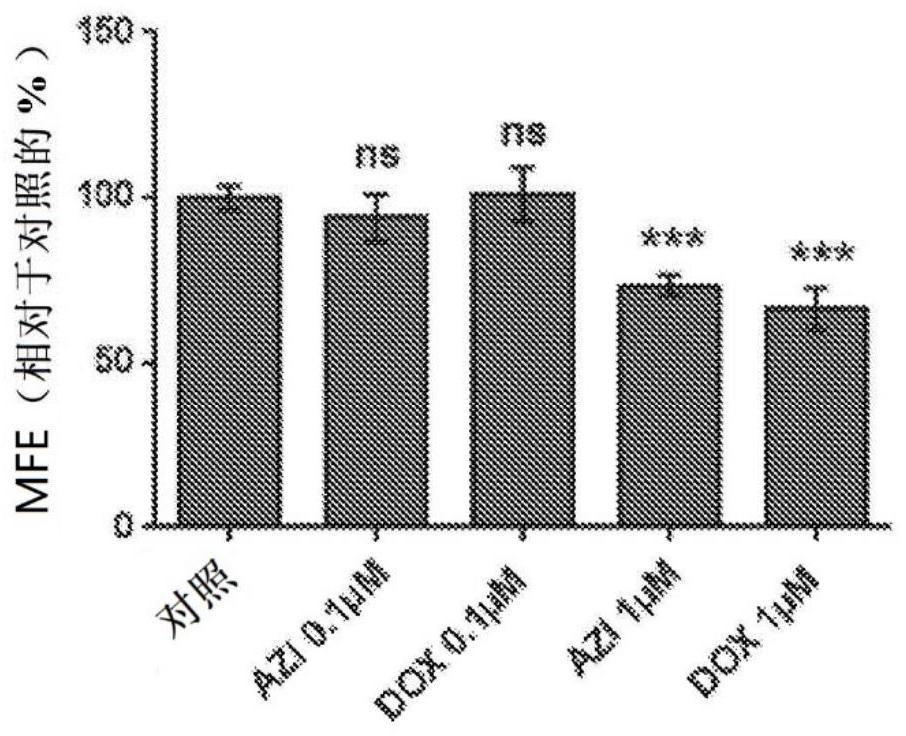
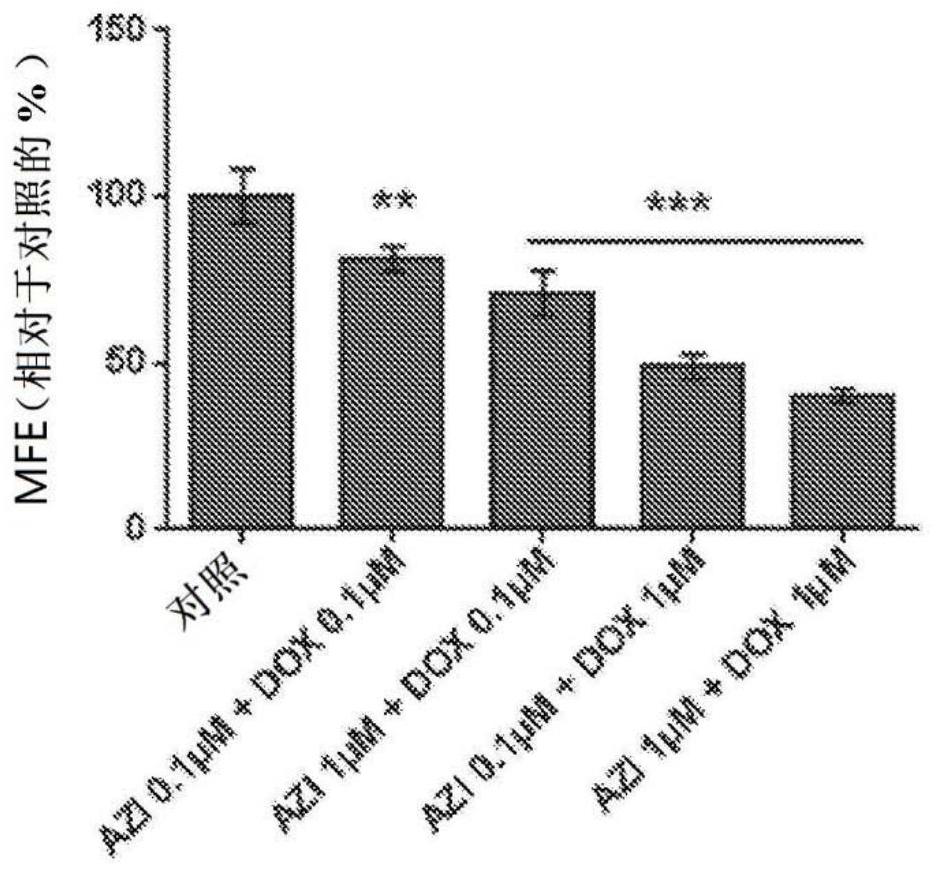
Triple combination therapies for Anti-aging
An anti-aging and therapeutic agent technology, applied in anti-tumor drugs, cosmetic preparations, medical preparations with inactive ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0105] The following compounds [12B], [13B] and [14B] show specific examples of conjugates of erythromycin family members according to this approach and use the general structure of the second series shown above. In compound [12B], R 1 fatty acid moiety The general structural substitution of , wherein "n" is an integer from 1 to 20, preferably 10 to 20, and other substitution positions have the normal components present on the structure of azithromycin. In compound [13B], R 2 has been substituted by the same general structure of the fatty acid moiety as in compound [12B], while another substituted position R 1 Has the normal constituents present on the structure of roxithromycin. As an example based on the second telithromycin conjugate formula, compound [14B] in R 1 have the same general structure of fatty acids, while NH-R 2 Conversely, the N(CH 3 ) 2 . In these examples, "n" is an integer of 1-20, preferably 10-20. For example, embodiments of erythromycin and fatt...
Embodiment 1
[0121] Example 1 - Conjugates of doxycycline and fatty acids. (4S,5S,6R,12aS)-4-(Dimethylamino)-3,5,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-9-(tetradecyl Acylamino)-4a,5,5a,6-tetrahydro-4H-tetracene-2-carboxamide (i.e., as described above in R 9 doxycycline conjugated to myristic acid and is shown below as compound [18]). 9-aminodoxycycline (prepared as described in Barden, Timothy C. et al. "Glycylcyclines". 3.9-Aminodoxycyclinecarboxamides. J. Med. Chem. 1994, 37, 3205-3211) (0.70 g, 1.5 mmol) , tetradecanoic acid (0.36g, 1.5mmol), HBTU (0.85g, 2.25mmol) and NMM (0.33ml, 3.0mmol) in the mixture of DCM (12ml) and DMF (4ml) solution in nitrogen atmosphere Stir at room temperature for 72 hours. The solvent was evaporated under reduced pressure. The resulting residue was triturated with acetonitrile (40ml) and the precipitate was collected by filtration, washed with acetonitrile (10ml), diethyl ether (20ml) and dried under vacuum. The crude product was dissolved in DMSO ...
Embodiment 2
[0123] Example 2 - Conjugates of doxycycline and fatty acids. (4S,5S,6R,12aS)-4-(Dimethylamino)-9-(hexadecanoylamino)-3,5,10,12,12a-pentahydroxy-6-methyl-1,11- Dioxo-4a,5,5a,6-tetrahydro-4H-tetracene-2-carboxamide. Compound [19] shown below was prepared according to the method in Example 1. LC-MS 698.2[M+H] + ,RT3.02min.
[0124]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com