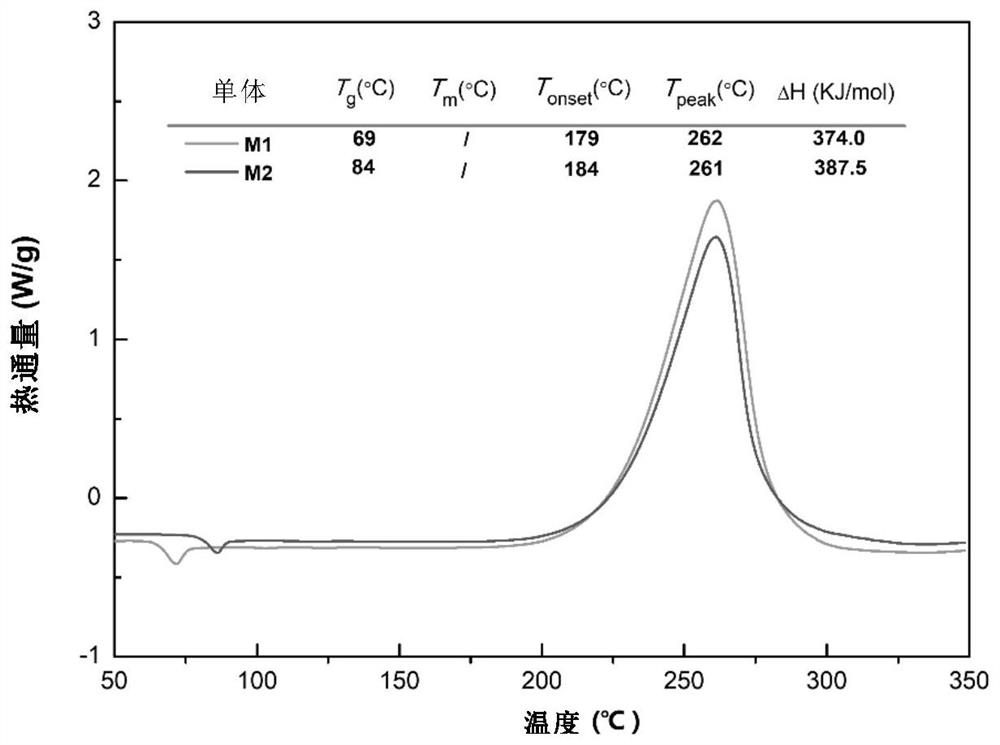
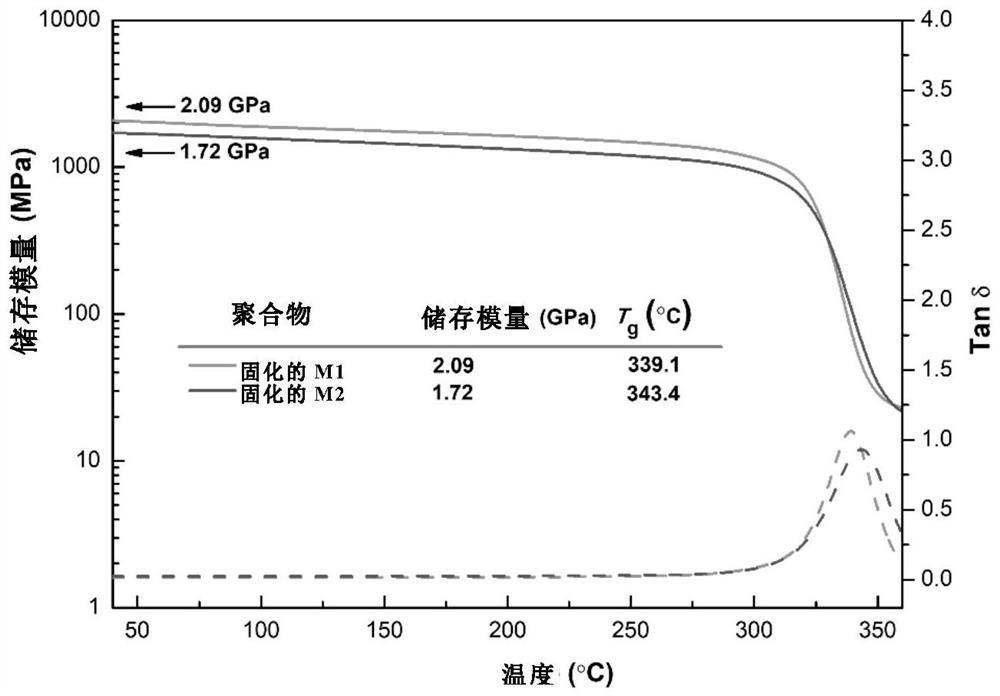
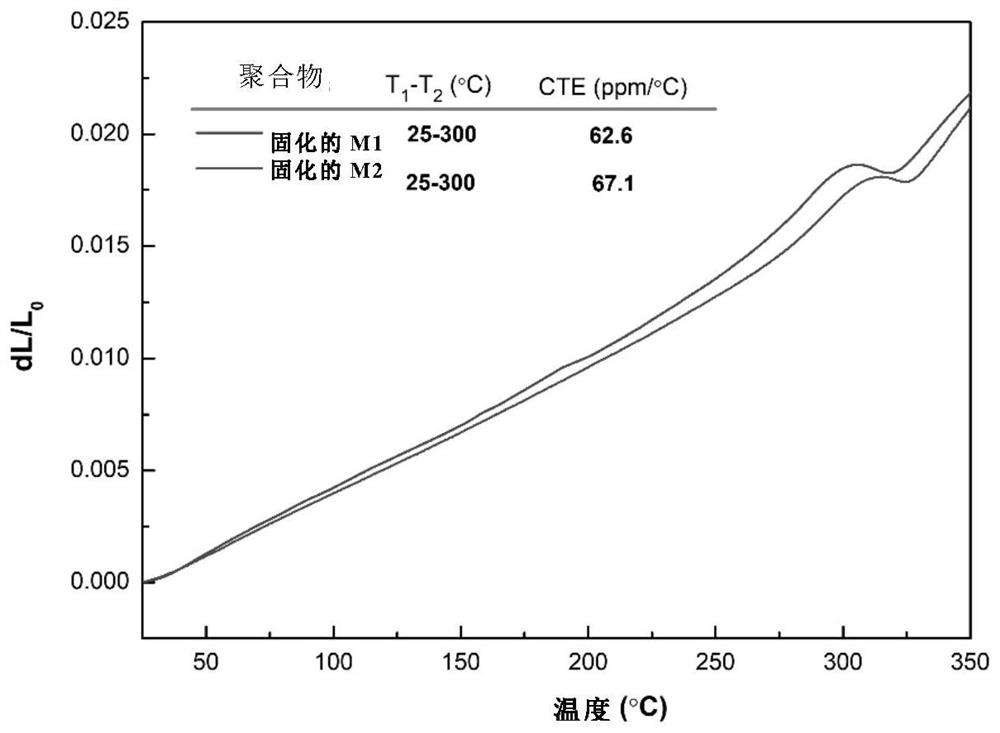
Synthesis method of molecular glass and application of molecular glass as high-frequency low-dielectric-constant material
A molecular glass and hydrogen atom technology, applied in organic chemistry and other fields, can solve problems such as uncontrollable polymer synthesis, thermal instability of thin films, aggregation and crystallization, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0216] Embodiment 1. Synthesis of double substituted 1,3,5-triazine S1
[0217]
[0218] Under the protection of nitrogen gas, add 180mmol magnesium chips, 100mL tetrahydrofuran and a grain of iodine (about 40mg) to a 250mL dry three-necked flask, then slowly add 120mmol 4-bromotoluene dropwise to keep the solution slightly boiling, and stir at reflux for 1h to obtain 4 - Methylphenyl Grignard reagent.
[0219] Take another 500mL dry three-necked flask, add 80mmol 2,4,6-trichloro-1,3,5-triazine under nitrogen protection, add 120mmol 4-methylbenzene Grignard reagent dropwise, and stir at -5°C Reacted for 19 hours. Quenched with ammonium chloride, extracted with ethyl acetate, concentrated to remove the solvent and followed by column chromatography to obtain 16.97 g of white solid compound 1 with a yield of 88%. 1 H NMR (CDCl 3 ,400MHz),δ(ppm)8.37(d,J=8.1Hz,2H),7.31(d,J=8.1Hz,2H),2.44(s,3H). 13 C NMR (CDCl 3 ,126MHz), δ(ppm)174.74,171.85,146.07,129.99,129.86,21.93.
[0...
Embodiment 2
[0223] Embodiment 2. Synthesis of Molecular Glass Monomer M1
[0224]
[0225] Take a 500mL single-necked bottle, add 13.2mmol intermediate S2, 48.0mmol NaOH and 80mL H 2 O, stirred at room temperature for 0.5h. Continue to add 120mL CHCl 3 , 24 mmol intermediate S1 and 0.13 mmol phase transfer catalyst cetyltrimethylammonium bromide. Raise the temperature to 60°C and react for 14h under vigorous stirring. After stopping the reaction, it was extracted with dichloromethane, dried, and the solvent was removed under reduced pressure. Column chromatography gave 7.70 g of white amorphous "molecular glass" monomer M1, yield: 75%.
[0226] 1 H NMR (500MHz, CDCl 3 )δ8.33(dd, J=11.6,8.3Hz,6H),8.10(s,2H),7.19(dd,J=15.0,5.0Hz,6H),7.13(dd,J=8.4,2.0Hz,2H ),7.06(dd,J=16.1,8.1Hz,4H),5.80(ddt,J=13.0,10.0,6.5Hz,2H),4.99–4.76(m,4H),3.31(d,J=6.3Hz, 4H), 3.11(s,8H), 2.31(s,6H), 1.72(s,6H).
[0227] 13 C NMR (CDCl 3 ,126MHz),δ(ppm)174.55,173.79,171.83,151.70,148.46,148.29,146.05,143.4...
Embodiment 3
[0228] Embodiment 3. the synthesis of intermediate S2'
[0229]
[0230] Take 250mL sealed tube, add 50mL acetone, 25mmol K 2 CO 3 , 10mmol of bisphenol AF, first stirred at room temperature for 30min, continued to add S2, and heated to 70°C for overnight reaction. After the reaction was stopped, the insoluble impurities were removed by suction filtration, and the solvent was spin-dried to obtain 4.11 g of the diallyl-substituted target intermediate as a colorless transparent liquid with a yield of 98.9%. The obtained intermediate was directly placed into a 50 mL single-necked flask, nitrogen was replaced 3 times, and reacted at 210° C. for 9 h. After the reaction was stopped, column chromatography was separated to obtain 3.771 g of light yellow liquid, yield: 91.7%.
[0231] 1 H NMR (CDCl 3 , 400MHz) δ(ppm) δ7.16(d, J=8.8Hz, 2H), 7.11(s, 2H), 6.80(d, J=8.5Hz, 2H), 5.97(m, 2H), 5.55(s ,2H,OH),5.12(dd,J=12.0,10.3Hz,4H),3.38(d,J=6.2Hz,4H). 19 F NMR (CDCl 3 , 376MHz) δ(...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com