Octapeptide and application thereof in preparation of medicine and health care product for improving memory
A technology for improving memory and health care products, applied in applications, medical preparations containing active ingredients, peptides, etc., can solve the problems of being easily degraded by proteases or polypeptide enzymes in the body, low bioavailability, and limited applications, etc., and achieve non-toxicity Effects of side effects, improvement of memory impairment, good safety and practicality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
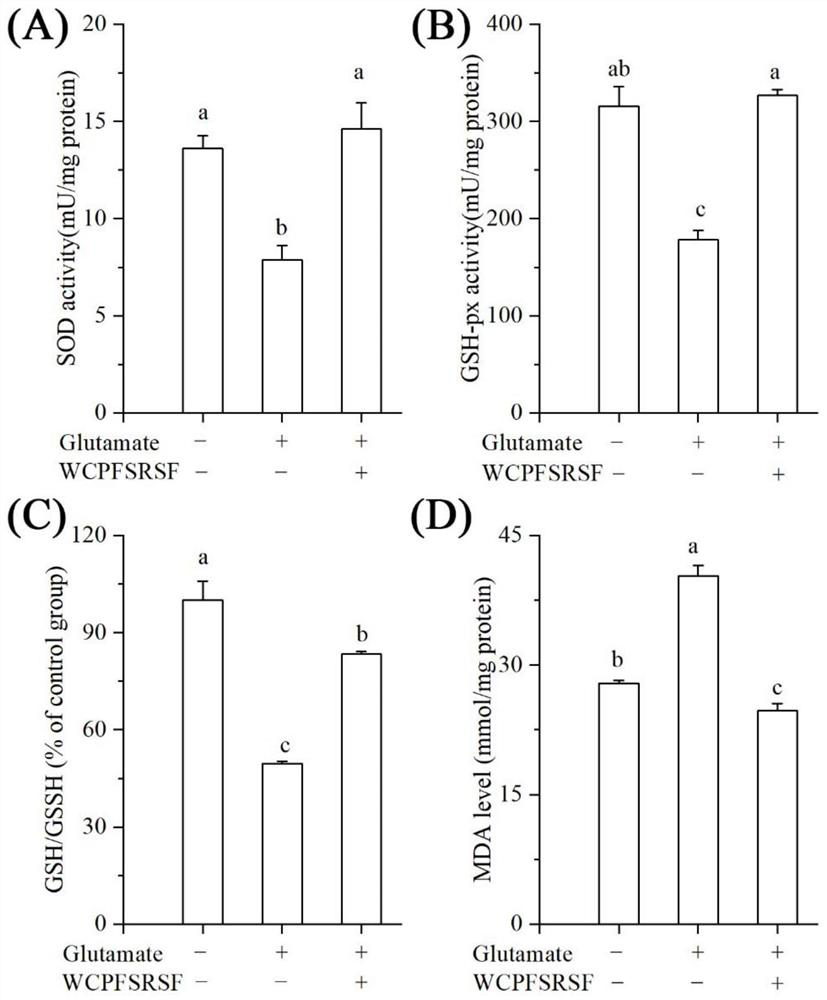
Examples
Embodiment 1
[0042] The synthesis of polypeptide WCPFSRSF by solid-phase synthesis includes the following steps: swelling and washing the dichloro resin, removing the Fmoc protecting group, adding the constituent amino acids of the polypeptide for condensation reaction, and repeating the process of removal-protection-condensation until all amino acids are connected . Purified by reverse-phase high-performance liquid chromatography to obtain pure polypeptide (>95%).
Embodiment 2
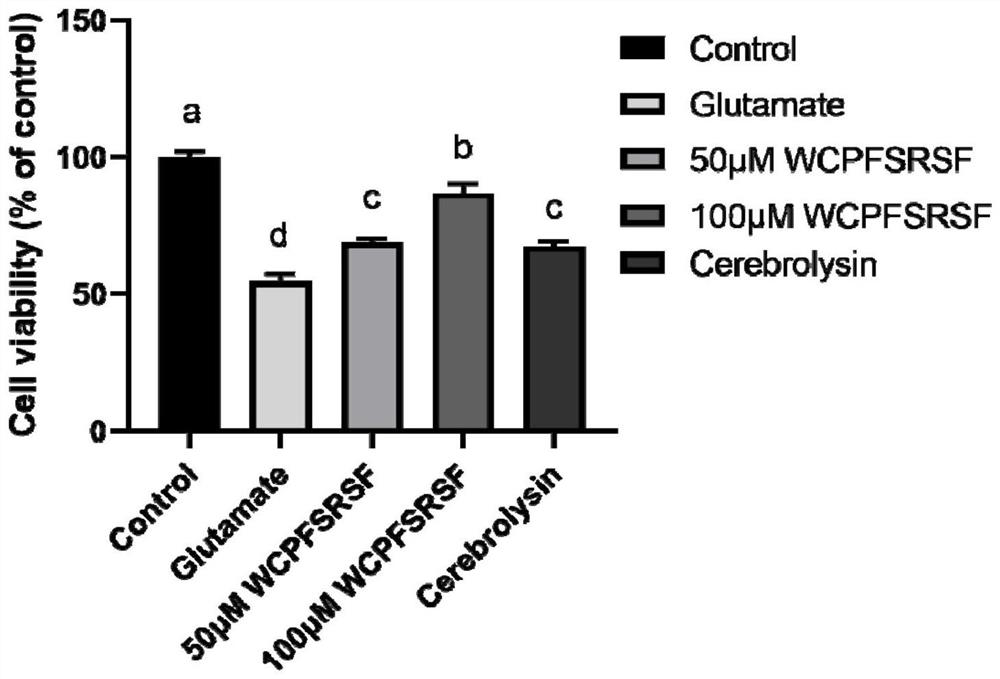
[0044] Effect of octapeptide on glutamate-induced neuronal cell survival. Such as figure 1 As shown, the cell survival rates of 50 μM and 100 μM WCPFSRSF treatment were 69.03±1.21% and 87.00±3.21%, respectively. The drug of positive control: the survival rate of cells treated with cerebrolysin (0.5 mg / mL) was 67.58±1.79%.
[0045] The test results show that the octapeptide WCPFSRSF has a better neuroprotective effect.
Embodiment 3
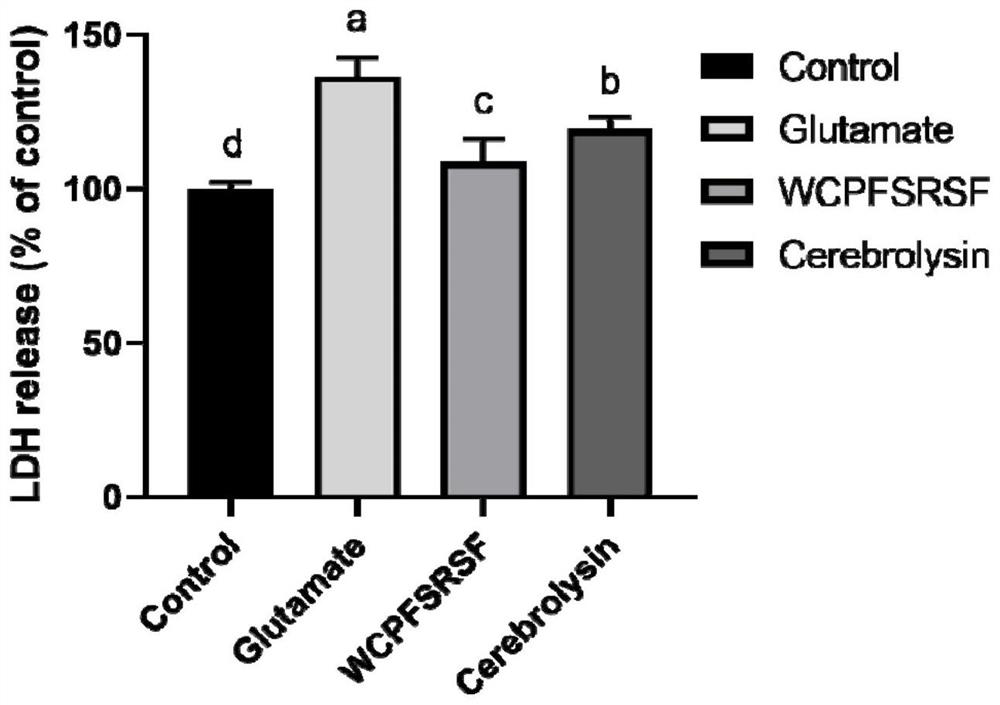
[0047] Determination of lactate dehydrogenase (lactate dehydrogenase, LDH)
[0048] SH-SY5Y cells were divided into 2×10 5Cells / mL were inoculated in a transparent 96-well plate, and culture medium was added. Six wells were set up for each experimental group, and blank wells and control wells were set up at the same time. After 24 hours, the medium was discarded, the model group was replaced with a medium containing 37.5mM glutamic acid, and the sample group was replaced with a medium containing both glutamic acid and samples, while cerebrolysin (0.5mg / mL) was used as a positive control , the blank group and the control group were replaced with fresh medium, and cultured for 24 hours. The culture was terminated before the detection, the supernatant was taken, and the lactate dehydrogenase kit was used for detection according to the instructions.
[0049] Such as figure 2 As shown, the test results showed that the LDH release rate of the glutamic acid treatment group was 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com