Jatrophane type diterpene derivative as well as preparation method and application thereof
A technology of elemane and its derivatives, applied in the field of pseudolelemane-type diterpene derivatives and their preparation, can solve the problems of increased drug efflux and formation of drug resistance, and achieve simple operation, mild reaction conditions, and high production efficiency. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
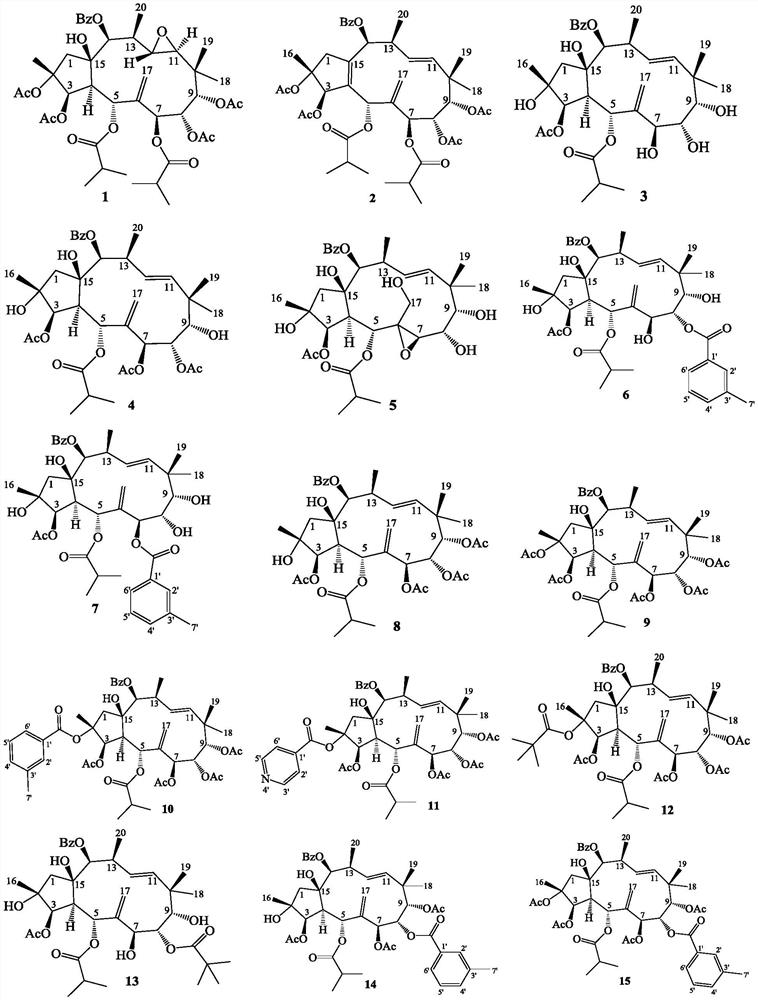
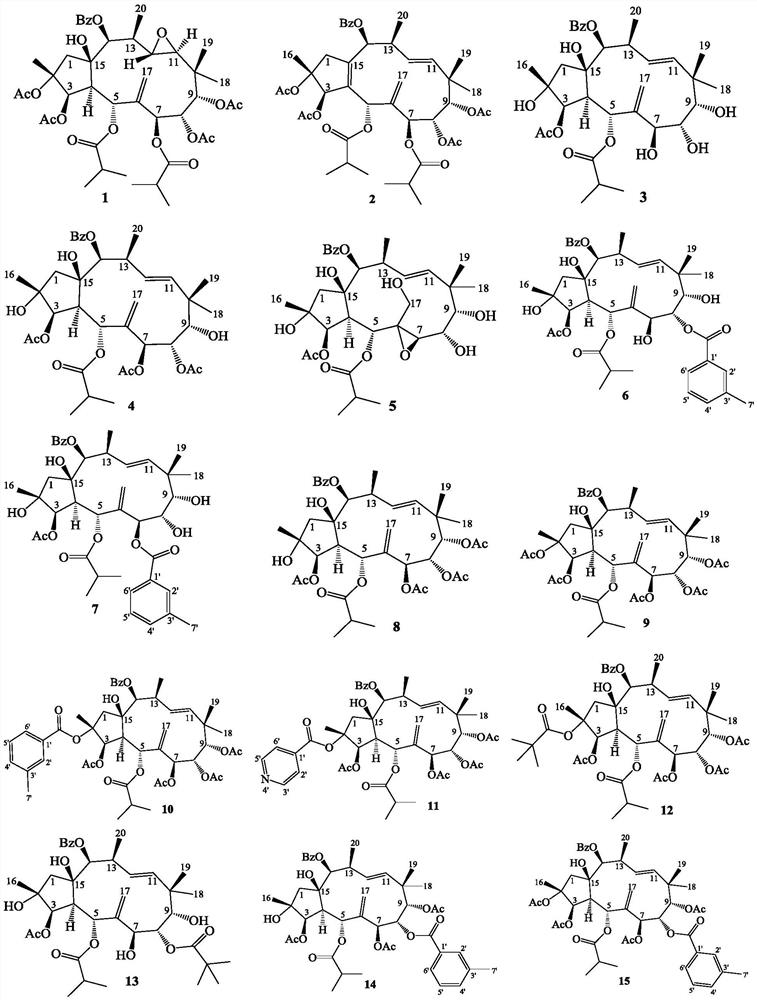
[0052] The name of the prepared compound 1 is: (2R, 3R, 4S, 5R, 7S, 8S, 9S, 11S, 12S, 13S, 14S, 15R)-14-benzoyloxy-15-hydroxyl-11,12-epoxy -5,7-Diisobutyryloxy-2,3,8,9-tetraacetoxy-pseudolevan-6(17)-ene:
[0053] Convert ES3 to 14β-benzoyloxy-15β-hydroxy-5α,7β-diisobutyryloxy-2α,3β,8α,9α-tetraacetoxy-pseudolemane-6(17),11E -diene 81mg, 0.10mmol was dissolved in dichloromethane 2mL, m-chloroperoxybenzoic acid 41mg, 0.20mmol, 85%, was added, stirred at room temperature for 6 days, quenched with saturated sodium thiosulfate, extracted with ethyl acetate, followed by Wash with saturated sodium bicarbonate, water, and saturated sodium chloride, dry over anhydrous sodium sulfate, concentrate the organic phase with ethyl acetate, and then pass through a silica gel column for 200-300 mesh gradient elution, and the eluent is petroleum at a volume ratio of 3:1 Ether and ethyl acetate, the white solid compound 1 name is: (2R, 3R, 4S, 5R, 7S, 8S, 9S, 11S, 12S, 13S, 14S, 15R)-14-benzoylox...
Embodiment 2
[0056] The name of the prepared compound 2 is: (2R,3R,5S,7S,8S,9S,13S,14S)-14-benzoyloxy-5,7-diisobutyryloxy-2,3,8,9 -Tetraacetoxy-pseudolemane-6(17),4E,11E-triene:
[0057] Convert ES3 to 14β-benzoyloxy-15β-hydroxy-5α,7β-diisobutyryloxy-2α,3β,8α,9α-tetraacetoxy-pseudolemane-6(17),11E -diene 46mg, 0.056mmol dissolved in toluene 3mL, add thionyl chloride 0.1mL, nitrogen protection, heat to 70°C, react with stirring for 1 hour, add saturated sodium bicarbonate to quench, ethyl acetate extract, water, saturated Wash with sodium chloride, dry over anhydrous sodium sulfate, concentrate the organic phase with ethyl acetate, and then pass through a silica gel column for 200-300 mesh gradient elution, and the eluent is petroleum ether and ethyl acetate at a volume ratio of 5:1, to obtain The name of colorless crystal 2 is: (2R,3R,5S,7S,8S,9S,13S,14S)-14-benzoyloxy-5,7-diisobutyryloxy-2,3,8, 9-tetraacetoxy-pseudolevane-6(17), 4E,11E-triene 40mg, yield 89%;
[0058] Mass spectrum and...
Embodiment 3
[0060] The name of the prepared compound 3 is: 14β-benzoyloxy-2α, 7β, 8α, 9α, 15β-pentahydroxy-5α-isobutyryloxy-3β-acetoxy-pseudolemane-6(17) ,11E-diene
[0061]Convert ES3 to 14β-benzoyloxy-15β-hydroxy-5α,7β-diisobutyryloxy-2α,3β,8α,9α-tetraacetoxy-pseudolemane-6(17),11E -diene 842mg, 1.03mmol was dissolved in methanol 10mL, 1,8-diazabicycloundec-7-ene 318mg, 2.09mmol was added, stirred at room temperature for 3.5 hours, hydrochloric acid 1mmol / L was added, 3mL was quenched, acetic acid Extracted three times with ethyl ester, washed with water, saturated sodium bicarbonate, and saturated sodium chloride successively, dried over anhydrous sodium sulfate, concentrated the organic phase with ethyl acetate, and then eluted through a silica gel column with a gradient of 200-300 mesh. The eluent was Petroleum ether and ethyl acetate at a volume ratio of 1:2, the white solid compound 3 is named 14β-benzoyloxy-2α,7β,8α,9α,15β-pentahydroxy-5α-isobutyryloxy- 3β-Acetoxy-Pseudoolemane-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com