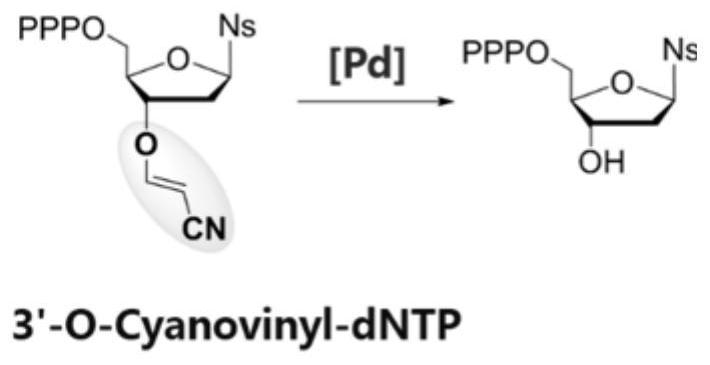
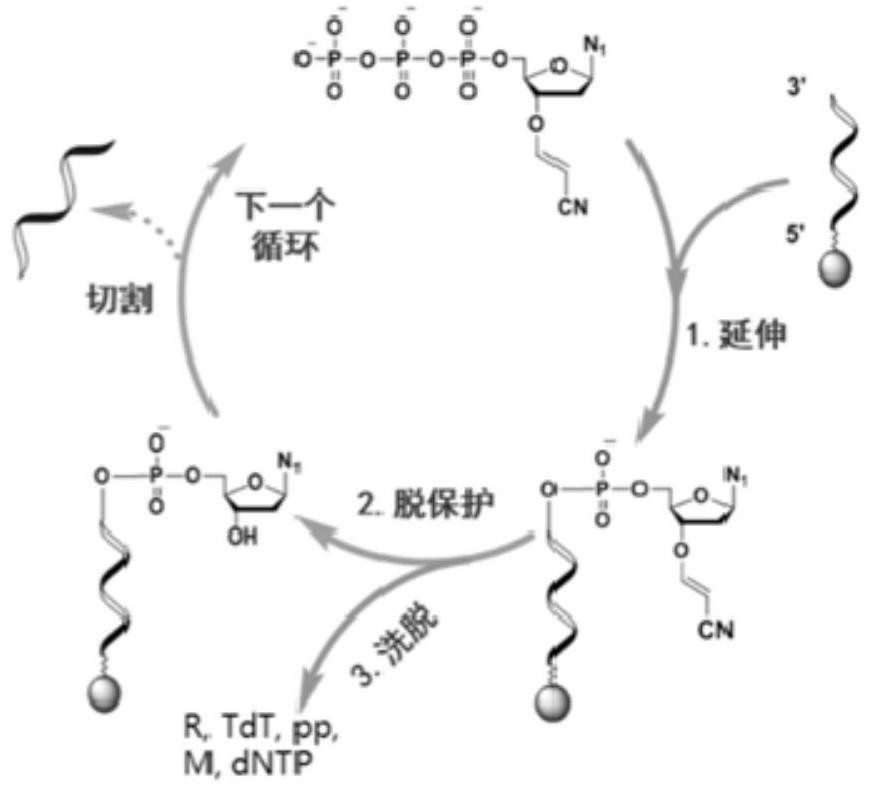
3'-O-reversibly closed nucleotide and application thereof in template-free enzymatic nucleic acid synthesis
A nucleotide and nucleic acid technology, applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve the problems of low deprotection efficiency, low catalytic extension rate, and inability to meet industrial DNA synthesis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
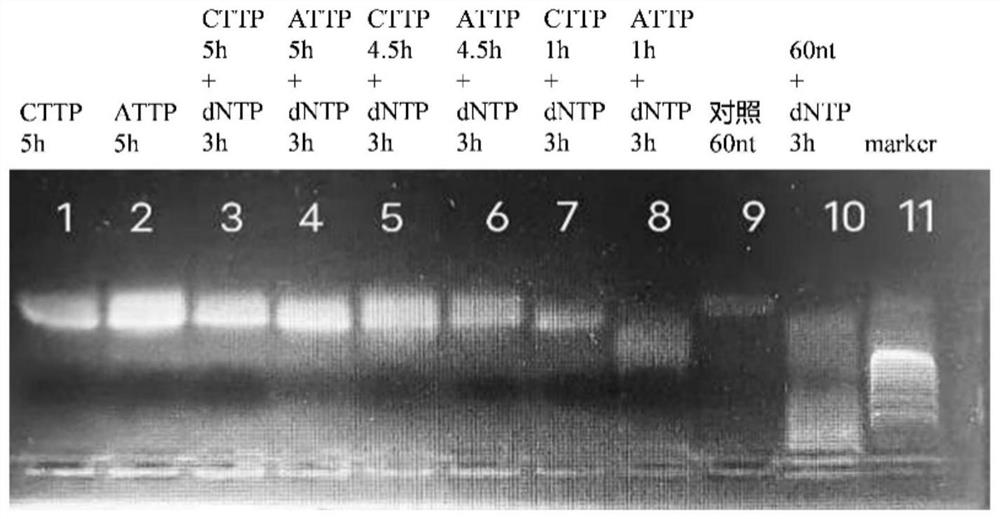
[0057] Embodiment 1, TdT enzymatic DNA extension experiment
[0058] (1) Experimental materials
[0059] 1. OligoDNA sequence: 5'-GCAGA TAATA CGACT CACTA TAGGG ATTTA GACTA CCCCAAAAAC GAAGG GGACT AAAAC-3' (60nt, SEQ ID NO.2);
[0060] 2. TdT (0.12mg / mL) (SEQ ID NO.1);
[0061] 3. TdT reaction buffer: 10×TdT reaction buffer, 10×CoCl 2 (2.5mM) (NEB, B0315S), where 1×TdT buffer: 20mM Tris-Acetate, 50mM KAc, 10mM MgAc 2 ,pH 7.9@25℃;
[0062] 4. Substrate: 3'-O-cyanovinyl-dTTP (CTTP, 10mM), 3'-O-Azidomethyl-dTTP (ATTP, 10mM), dNTP mix (dNTP, 10mM);
[0063] 5. ddH 2 O;
[0064] (2) Experimental operation
[0065] (1) Configure four TdT enzymatic DNA extension reaction systems:
[0066] oligoDNA (5μL, 2.5mM, 60nt), 10×TdT reaction buffer (5μL), 2.5mM CoCl 2 Solution (5μL), 8.3μL TdT (0.12mg / mL), and the corresponding nucleotide substrate were added to a microcentrifuge tube (1.5mL), and ddH 2 O dilute the mixture to 50 μL;
[0067]
[0068]
[0069] (2) with ddH 2 O...
Embodiment 2
[0075] Embodiment 2, deprotection experiment
[0076] (1) Experimental materials
[0077] Deprotection reagents: 0.5M TCEP (pH=10) solution for ATTP substrate, 0.05M diacetonitrile palladium dichloride (PdCl 2 (CH 3 EN) 2 ) solution is used for CTTP substrate;
[0078] (2) Experimental operation
[0079] (1) Configure four TdT enzymatic DNA extension reaction systems:
[0080] oligoDNA (15μL, 2.5mM, 60nt), 10×TdT reaction buffer (15μL), 2.5mM CoCl 2 Solution (15μL), 16.6μL TdT (0.12mg / mL), and the corresponding nucleotide substrate were added to a microcentrifuge tube (1.5mL), and washed with ddH 2 O dilute the mixture to 150 μL;
[0081] # Substrate and system Volume (μL) 1 CTTP 1.5 2 ATTP 1.5 3 dNTP 12 4 blank --(no added)
[0082] (2) The mixture was incubated at 37°C for 1 hour, 4.5 hours and 5 hours according to different substrates;
[0083] (3) Take out the reaction solution, add 2.5 μL 0.5M TCEP (pH=10) solution to...
Embodiment 3、3
[0090] The synthesis of embodiment 3,3'-O-cyanovinyl-dNTP
[0091] Using 5'-OTBDMS-protected nucleosides as raw materials, firstly through a condensation reaction, the 3'-OH is selectively directly esterified with formic acid to generate the corresponding methyl ester. Utilizing the wittig reactivity of methyl ester, react with phosphorus ylide reagent (triphenylphosphoranylidene) acetonitrile to generate 3'-O-cyanovinyl, and obtain 3'-O-cyanovinyl after removal of 5'-OTBDMS -dNTP precursor, followed by triphosphorylation and deprotection to synthesize the desired substrate. Its specific synthetic flow chart is as Figure 5 Shown ((i) HCOOH, EDCI, DMAP, DCM, 0℃-room temperature, 6h; (ii) (1) 2-(triphenylphosphaneylidene) acetonitrile (Wittigreagents), Toluene, 120℃, 9h; (2) TBAF·3H 2 O, THF, room temperature, 1h; (iv) (1) POCl 3 ,1,8-Bis(dimethyl amino)naphthalene,(MeO) 3 PO, 0℃, 2h; (2) Tributylammoniumpyrophosphate, Bu 3 N,CH 3 CN, 0℃, 10min; (3)NH 4 OH, 25°C, 16h). ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com