Liquid phase synthesis method of itkatide
A synthesis method and itkatide technology, applied in the field of peptide synthesis, can solve problems such as cumbersome steps, and achieve the effects of easy availability of raw materials, convenient operation and easy control of purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] As mentioned in the background art, in the existing solid-phase synthesis method, the disulfide bond of itkatide is easily misconnected, and the purity of the crude product is low, while the existing liquid-phase synthesis method has relatively cumbersome steps. In order to improve this situation, in a typical embodiment of the present application, a liquid-phase synthesis method of itkatide is provided, the synthesis method comprising:
[0042] S101, using Cbz as a protecting group to sequentially synthesize the sequence of formula (I) in liquid phase,
[0043] Ac- D -Cys(K)- D -Ala- D -Arg(Pbf)- D -Arg(Pbf)- D -Arg(Pbf)- D -Ala- D -Arg(Pbf)-NH 2 (I);
[0044] S102, removing Ac- in the sequence of formula (I) D -The side chain protecting group K of Cys(K) obtains the sequence of formula (II),
[0045] Ac- D -Cys- D -Ala- D -Arg(Pbf)- D -Arg(Pbf)- D -Arg(Pbf)- D -Ala- D -Arg(Pbf)-NH 2 (II);
[0046] S103, by Boc- L -Cys(S-S-Py)-OtBu in formula (II) s...
Embodiment 1
[0091] Example 1: Cbz- D -Arg(Pbf)-NH 2 Synthesis
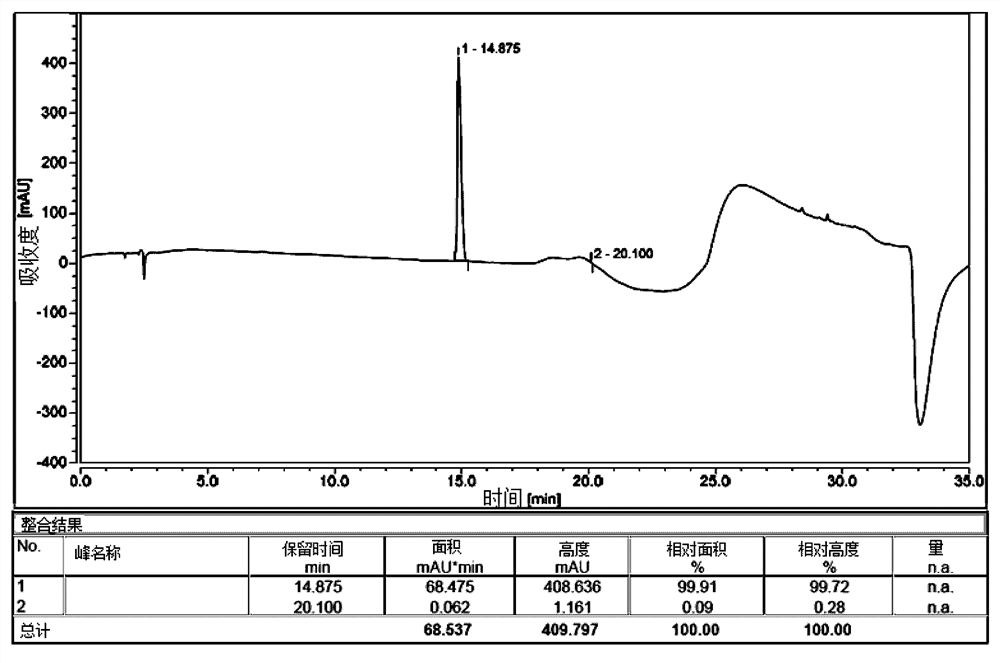
[0092] Cbz- D -Arg(Pbf)-OH (56.5g, 0.1mmol) was dissolved in DMAc (1.2L, 20V), Oxymapure (17.2g, 1.2eq); EDCI (23.2g, 1.2eq), DIPEA (19.4g, 1.5 eq), after the reaction solution was stirred for 30 minutes, ammonium acetate (38.5g, 5.0eq) was added, and the reaction was stirred at 26 degrees for 7.5h; HPLC monitored the reaction end point; after the reaction, the reaction solution was added dropwise with water (6.0L, 100 vol. ), stirring and crystallizing at 0-10°C for 2 hours; filtering, drying the filter cake and collecting 52.5g of material, with a purity of 99.5% and a yield of 93%.
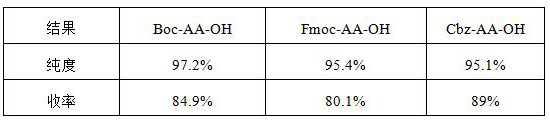
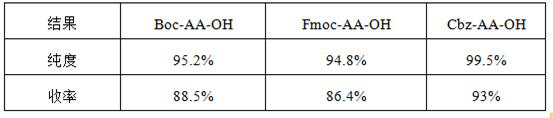
[0093] The condensation steps are operated in the same way, and only the amino acid protecting group of the amino acid raw material is changed to Boc or Fmoc protected amino acid; the yield and purity comparison of the obtained products are shown in the following table:
[0094] Table 2:
[0095]
Embodiment 2
[0096] Example 2: Cbz- D -Ala- D -Arg(Pbf)-NH 2 Synthesis
[0097] Under nitrogen protection, Cbz- D -Arg(Pbf)-NH 2 (52.5g, 1.0eq) was dissolved in DMAc (530ml, 10vol.), and Pd / C (5.3g, 0.1eq) was added to the reaction system; the reaction system was replaced by hydrogen six times; the reaction temperature was controlled at 20-30 degrees for 2 hours; The end point of the reaction was monitored by HPLC; after the reaction, the reaction system was replaced with nitrogen six times; filtered, the filter cake was soaked with DMAc (100 ml, 2vol.), and temporarily stored under the protection of nitrogen (the next hydrogenation reaction can still be used); the filtrate was directly for the next reaction.
[0098] Cbz- D -Ala-OH (20.8g, 1.0eq) was dissolved in DMAc (208ml, 10vol.), Oxymapure (13.22g, 1.0eq), EDCI (18.72g, 1.05eq), DIPEA (12.62g, 1.05eq) were added, After the reaction solution was stirred for 30 minutes, NH 2 - D -Arg(Pbf)-NH 2 DMAc solution (1.05eq), the reac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com