Orthopedic medical binding material with antibacterial effect and preparation method of medical binding material
An adhesive material and orthopaedic technology, applied in the field of medical materials, can solve the problems of small adhesive force, difficult to bond and fix bone fragments, poor water solubility, and high brittleness, and achieve excellent antibacterial and bactericidal effects, improve compression resistance, and good The effect of compressive strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
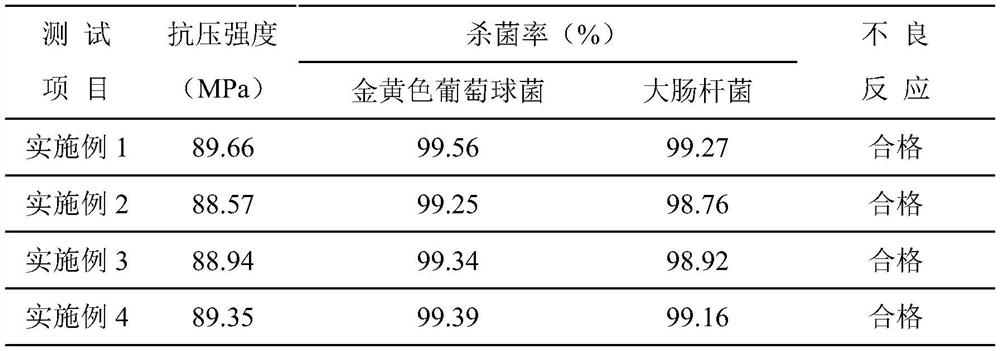
Examples
Embodiment 1
[0014] A preparation method of an orthopedic medical adhesive material with antibacterial effect, comprising the following raw materials in parts by weight: 45 parts of self-made modified calcium phosphate bone cement, 12 parts of n-octyl α-cyanoacrylate, 15 parts of dopamine, alginic acid 4 parts of sodium, 3 parts of lecithin, 5 parts of 3,4-dihydroxyphenylalanine, 11 parts of collagen, 8 parts of chitosan, 3 parts of arthine, 3 parts of phenylalanine, 3-p-chloro 3 parts of phenoxy-1,2-propanediol, 2.5 parts of β-cyclodextrin.
[0015] Wherein, the self-made modified calcium phosphate bone cement is prepared by the following method: adding calcium phosphate bone cement, L-alanine, β-cyclodextrin and butyl acrylate into a ball mill, then ball milling for 3 hours, and then adding two Methicone and sodium carboxymethylcellulose ball milled for 2 hours; calcium phosphate cement, L-alanine, β-cyclodextrin, butyl acrylate, dimethylsiloxane and sodium carboxymethylcellulose The ma...
Embodiment 2
[0021] A preparation method of an orthopedic medical adhesive material with antibacterial effect, comprising the following raw materials in parts by weight: 60 parts of self-made modified calcium phosphate bone cement, 18 parts of n-octyl α-cyanoacrylate, 24 parts of dopamine, alginic acid 10 parts of sodium, 6 parts of lecithin, 10 parts of 3,4-dihydroxyphenylalanine, 16 parts of collagen, 15 parts of chitosan, 5 parts of arthine, 5 parts of phenylalanine, 3-p-chloro 6 parts of phenoxy-1,2-propanediol, 5 parts of β-cyclodextrin.
[0022] Wherein, the self-made modified calcium phosphate bone cement is prepared by the following method: adding calcium phosphate bone cement, L-alanine, β-cyclodextrin and butyl acrylate into a ball mill, then ball milling for 5 hours, and then adding two Methicone and sodium carboxymethylcellulose ball milled for 3 hours; calcium phosphate cement, L-alanine, β-cyclodextrin, butyl acrylate, dimethylsiloxane and sodium carboxymethylcellulose The m...
Embodiment 3
[0028] A preparation method of an orthopedic medical adhesive material with antibacterial effect, comprising the following raw materials in parts by weight: 50 parts of self-made modified calcium phosphate bone cement, 14 parts of n-octyl α-cyanoacrylate, 18 parts of dopamine, alginic acid 6 parts of sodium, 4 parts of lecithin, 7 parts of 3,4-dihydroxyphenylalanine, 13 parts of collagen, 10 parts of chitosan, 4 parts of amino acid, 4 parts of phenylalanine, 3-p-chloro 4 parts of phenoxy-1,2-propanediol, 3 parts of β-cyclodextrin.
[0029] Wherein, the self-made modified calcium phosphate bone cement is prepared by the following method: adding calcium phosphate bone cement, L-alanine, β-cyclodextrin and butyl acrylate into a ball mill, then ball milling for 4 hours, and then adding two Methicone and sodium carboxymethylcellulose ball milled for 2.5 hours; calcium phosphate cement, L-alanine, β-cyclodextrin, butyl acrylate, dimethylsiloxane and sodium carboxymethylcellulose Th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Compressive strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com