A kind of high-intensity photoreversible adhesive, preparation method and application
A high-intensity light and adhesive technology, applied in the direction of adhesives, adhesive types, polyurea/polyurethane adhesives, etc., can solve the adverse effects of light-induced reversible adhesive bonding strength, freezing of polymer chains, and inability to react and other problems, to achieve strong reactivity and operability, strong bonding strength and stability, and improve the effect of bonding strength and creep resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0131] Adhesive structural formula:
[0132]
[0133] 0
[0134] A is
[0135]
[0136] B is:
[0137]
[0138] a=19
[0139] D is:
[0140]
[0141] b=4
[0142] R is
[0143]
[0144] Preparation methods include:
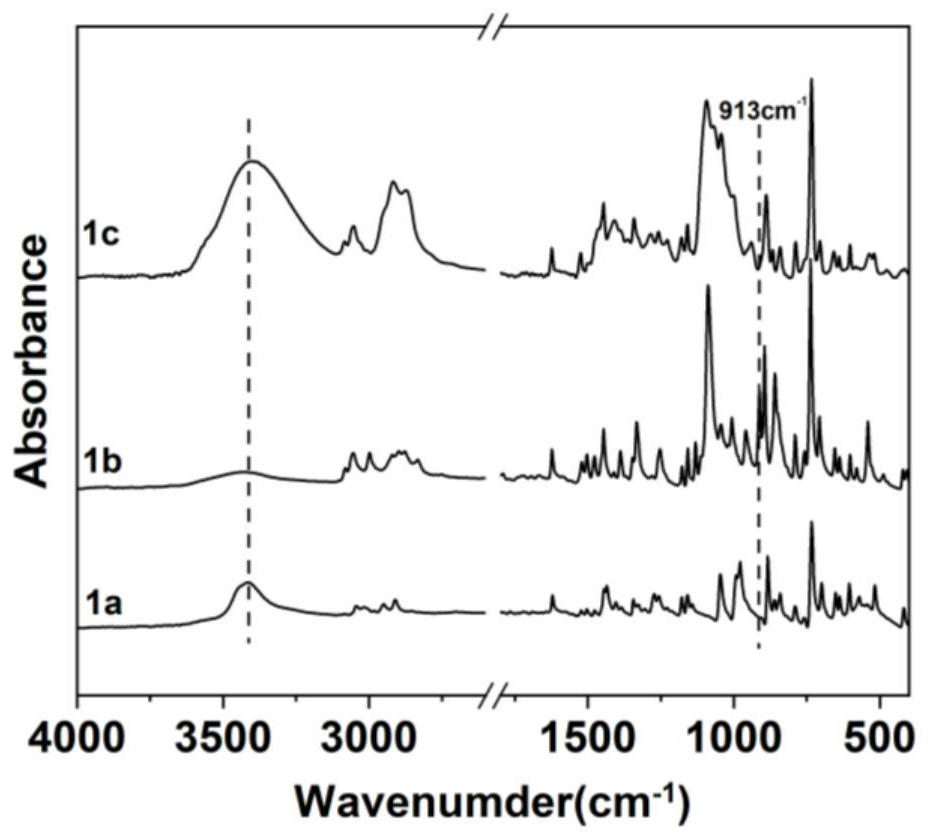
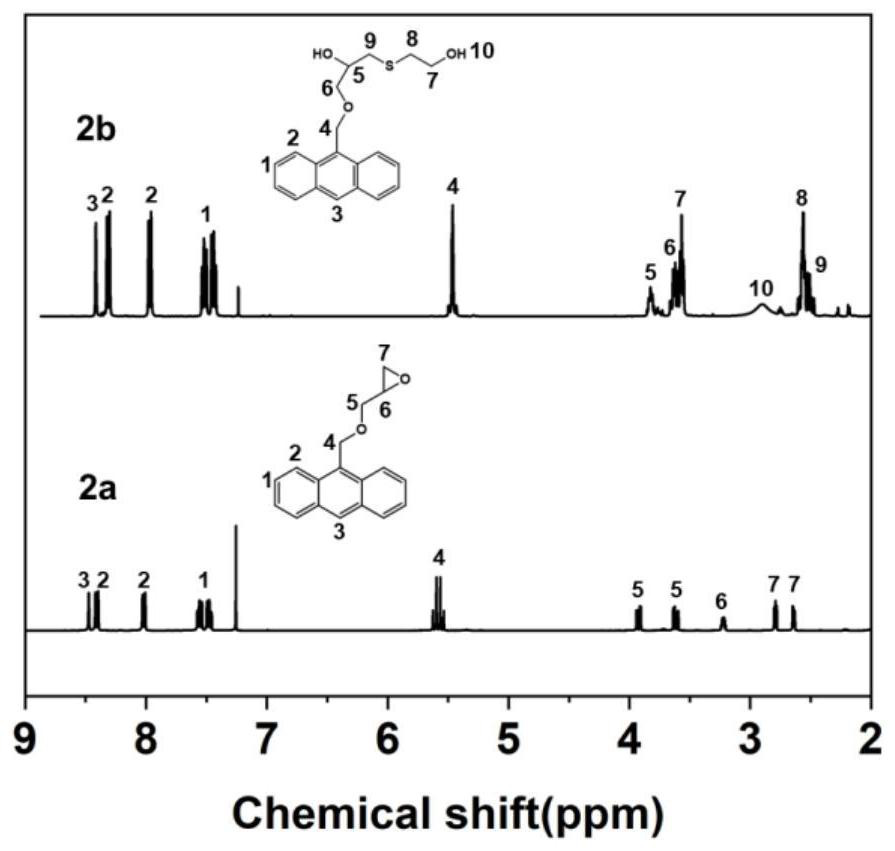
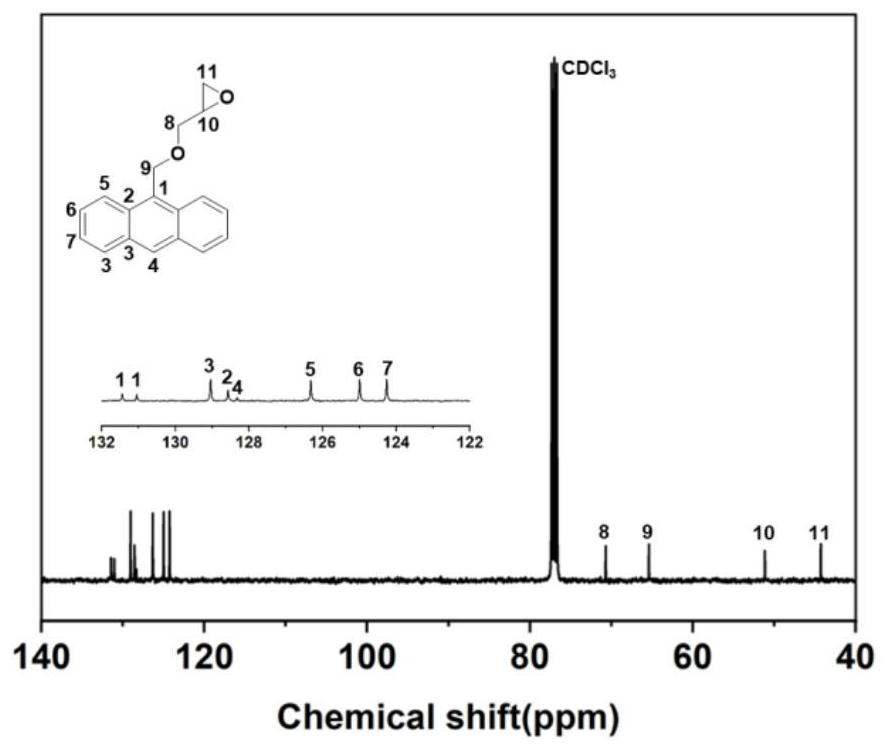
[0145] 1) Preparation of anthracene-containing epoxidation products: Dissolve 1 mol of 9-anthracene methanol (AM) raw material in 25 mol of non-polar solvent toluene, then add 8 mol of epichlorohydrin, keep the temperature in a water bath at 65°C, and then add 3 mol of of sodium hydroxide and 0.02 mol of tetramethylammonium bromide (TMAB). Insulated and stirred for 4 hours, centrifuged, toluene extracted, rotary evaporated and vacuum dried to obtain an epoxidized product containing anthracenyl groups——9-anthracenemethanol epoxide (AER);
[0146] 2) Preparation of double-terminal hydroxyl compounds containing anthracenyl side chains: Step 1) Mix 0.5 mol of 9-anthracene methano...
Embodiment 2
[0175] The structural formula of adhesive is the same as embodiment 1.
[0176] Preparation methods include:
[0177] 1) Preparation of anthracene-containing epoxidation products: Dissolve 1 mol of 9-anthracene methanol (AM) raw material in 20 mol of non-polar solvent toluene, then add 5 mol of epichlorohydrin, keep the temperature in a water bath at 60°C, and then add 2 mol of Sodium hydroxide and 0.01 mol of tetramethylammonium bromide (TMAB). Insulated and stirred for 6 hours, centrifuged, toluene extracted, rotary evaporated and vacuum dried to obtain an epoxidized product containing anthracenyl groups—9-anthracenemethanol epoxide (AER);
[0178] 2) Preparation of double-terminal hydroxyl compounds containing anthracenyl side chains: Step 1) Mix 0.5 mol of 9-anthracene methanol epoxide with 0.5 mol mercaptoethanol, dissolve in 10 mol toluene, and add DMP in 0.5% of the mass fraction -30 promoter, after stirring evenly, react at room temperature (20°C) for 3h, and obtain ...
Embodiment 3
[0206] Adhesive structural formula is the same as embodiment 1.
[0207] Preparation methods include:
[0208] 1) Preparation of anthracene-containing epoxidation products: Dissolve 1 mol of 9-anthracene methanol (AM) raw material in 30 mol of non-polar solvent toluene, then add 10 mol of epichlorohydrin, keep the temperature in a water bath at 70°C, and then add 4 mol of of sodium hydroxide and 0.03 mol of tetramethylammonium bromide (TMAB). Insulated and stirred for 6 hours, centrifuged, toluene extracted, rotary evaporated and vacuum dried to obtain an epoxidized product containing anthracenyl groups - 9-anthracenemethanol epoxide (AER);
[0209] 2) Preparation of double-terminal hydroxyl compounds containing anthracenyl side chains: Step 1) Mix 0.5mol of 9-anthracene methanol epoxide with 0.55mol mercaptoethanol, dissolve in 15mol toluene, add DMP with 1% of the mass fraction -30 promoter, after stirring evenly, react at room temperature (30°C) for 2h, and obtain an epox...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com