High-strength photoreversible adhesive, and preparation method and application thereof
A high-intensity light, adhesive technology, applied in the direction of adhesives, adhesive types, polyurea/polyurethane adhesives, etc., can solve the adverse effects of polymer segment freezing, inability to react, and light-induced reversible adhesive bond strength And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0131] Adhesive structural formula:
[0132]
[0133] 0
[0134] A is
[0135]
[0136] B is:
[0137]
[0138] a=19
[0139] D is:
[0140]
[0141] b=4
[0142] R is
[0143]
[0144] Preparation methods include:
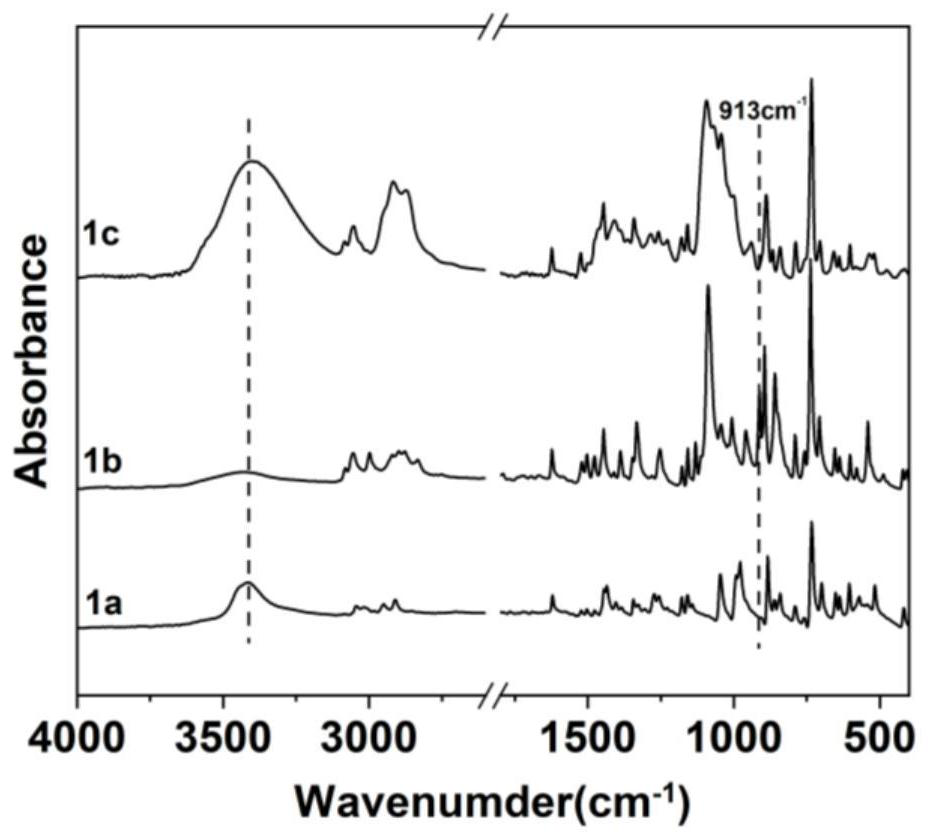
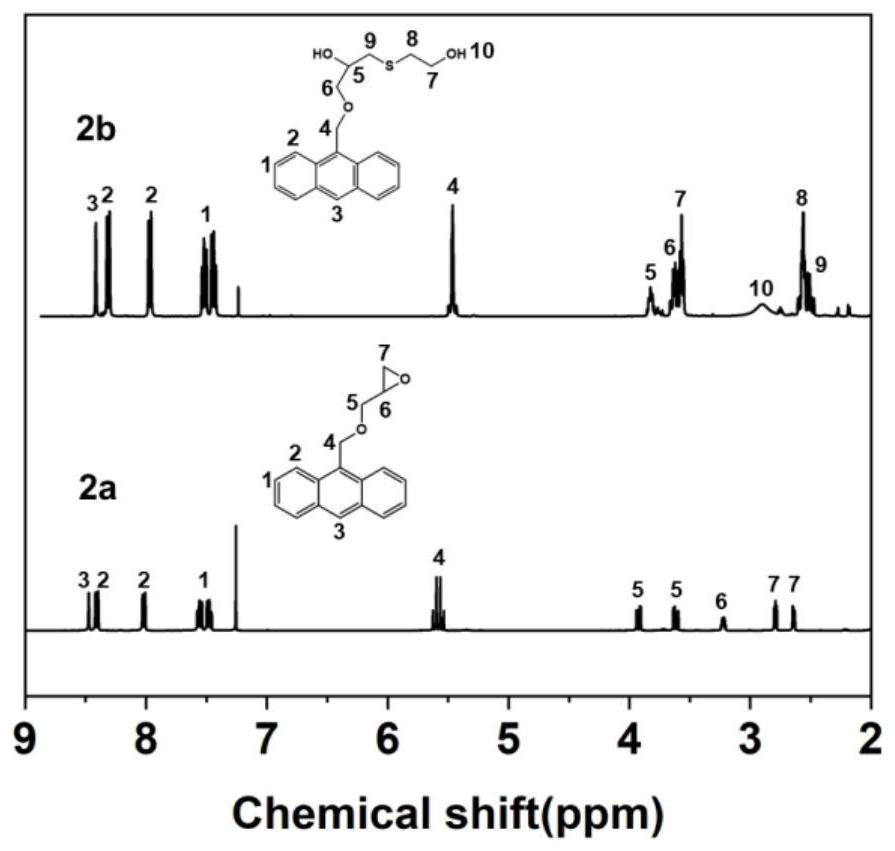
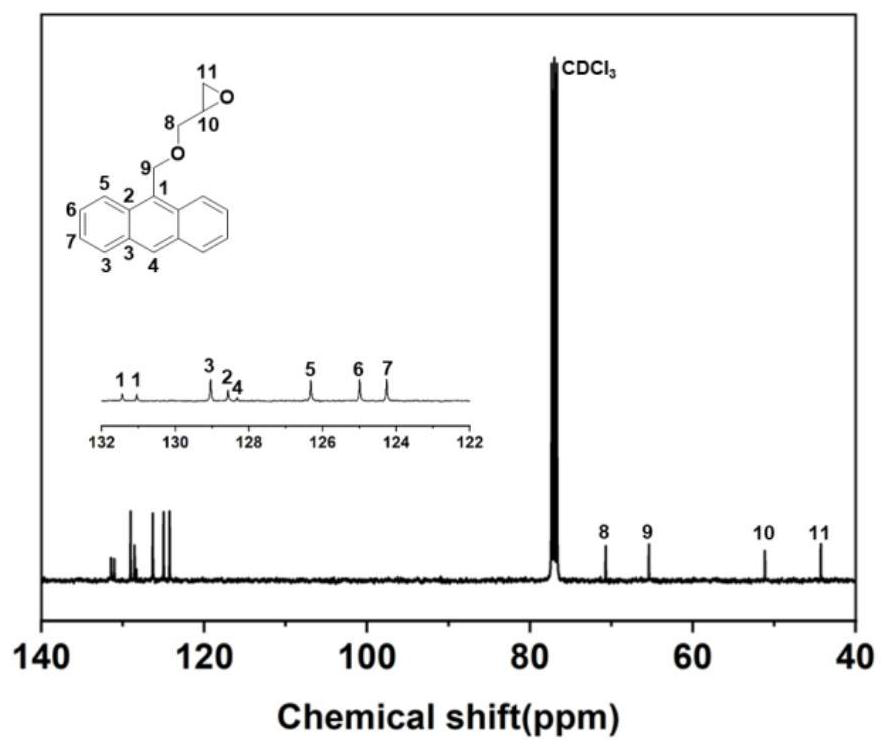
[0145] 1) Preparation of anthracene-containing epoxidation products: Dissolve 1 mol of 9-anthracene methanol (AM) raw material in 25 mol of non-polar solvent toluene, then add 8 mol of epichlorohydrin, keep the temperature in a water bath at 65°C, and then add 3 mol of of sodium hydroxide and 0.02 mol of tetramethylammonium bromide (TMAB). Insulated and stirred for 4 hours, centrifuged, toluene extracted, rotary evaporated and vacuum dried to obtain an epoxidized product containing anthracenyl groups——9-anthracenemethanol epoxide (AER);
[0146] 2) Preparation of double-terminal hydroxyl compounds containing anthracenyl side chains: Step 1) Mix 0.5 mol of 9-anthracene methano...
Embodiment 2
[0175] The structural formula of adhesive is the same as embodiment 1.
[0176] Preparation methods include:
[0177] 1) Preparation of anthracene-containing epoxidation products: Dissolve 1 mol of 9-anthracene methanol (AM) raw material in 20 mol of non-polar solvent toluene, then add 5 mol of epichlorohydrin, keep the temperature in a water bath at 60°C, and then add 2 mol of Sodium hydroxide and 0.01 mol of tetramethylammonium bromide (TMAB). Insulated and stirred for 6 hours, centrifuged, toluene extracted, rotary evaporated and vacuum dried to obtain an epoxidized product containing anthracenyl groups—9-anthracenemethanol epoxide (AER);
[0178] 2) Preparation of double-terminal hydroxyl compounds containing anthracenyl side chains: Step 1) Mix 0.5 mol of 9-anthracene methanol epoxide with 0.5 mol mercaptoethanol, dissolve in 10 mol toluene, and add DMP in 0.5% of the mass fraction -30 promoter, after stirring evenly, react at room temperature (20°C) for 3h, and obtain ...
Embodiment 3
[0206] Adhesive structural formula is the same as embodiment 1.
[0207] Preparation methods include:
[0208] 1) Preparation of anthracene-containing epoxidation products: Dissolve 1 mol of 9-anthracene methanol (AM) raw material in 30 mol of non-polar solvent toluene, then add 10 mol of epichlorohydrin, keep the temperature in a water bath at 70°C, and then add 4 mol of of sodium hydroxide and 0.03 mol of tetramethylammonium bromide (TMAB). Insulated and stirred for 6 hours, centrifuged, toluene extracted, rotary evaporated and vacuum dried to obtain an epoxidized product containing anthracenyl groups - 9-anthracenemethanol epoxide (AER);
[0209] 2) Preparation of double-terminal hydroxyl compounds containing anthracenyl side chains: Step 1) Mix 0.5mol of 9-anthracene methanol epoxide with 0.55mol mercaptoethanol, dissolve in 15mol toluene, add DMP with 1% of the mass fraction -30 promoter, after stirring evenly, react at room temperature (30°C) for 2h, and obtain an epox...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com