Recombinant hirudin fusion protein with targeting and long-acting functions and coding gene and application thereof
A technology of recombinant hirudin and fusion protein, which is applied in the biological field and can solve problems such as hindering the long-term cycle characteristics of albumin and large molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1, the design of hirudin prodrug fusion protein
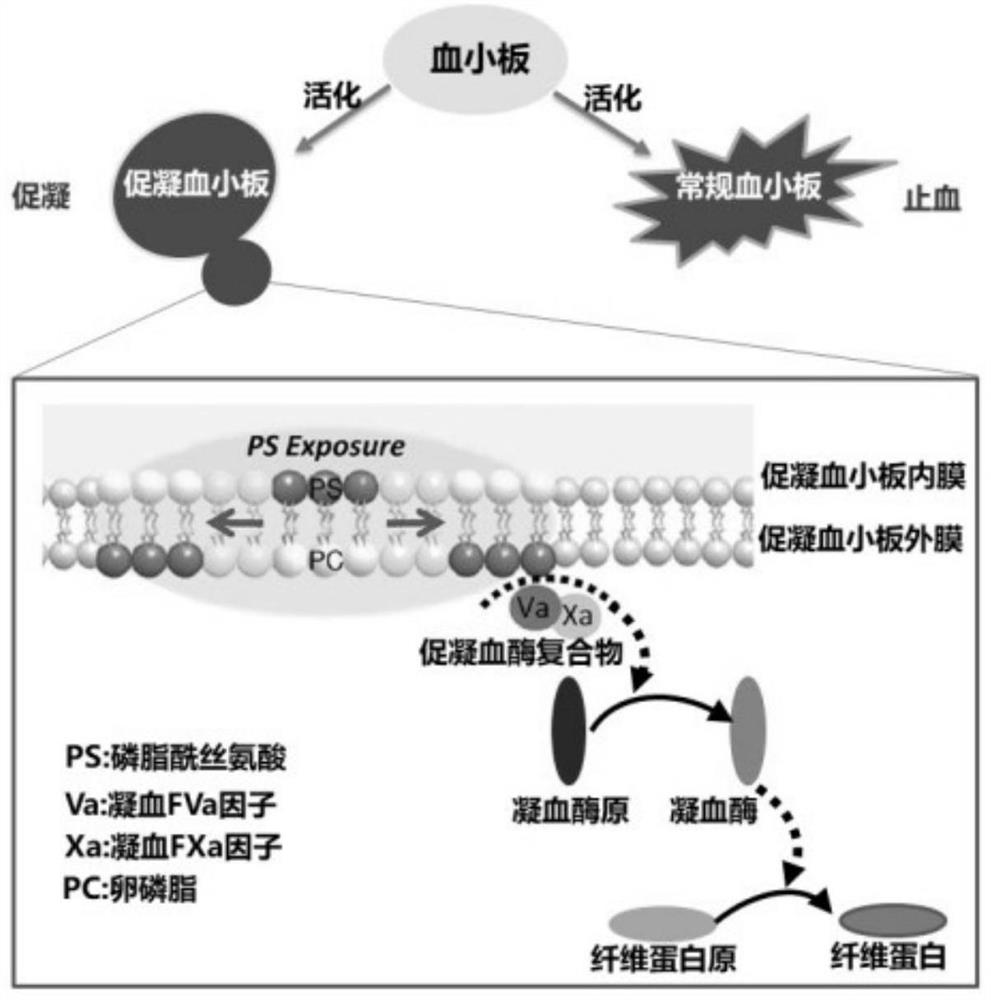
[0057] The purpose of the present invention is to solve the problems of high bleeding risk and short half-life of hirudin. Based on this, the present invention designs a "targeted long-acting hirudin prodrug" composed of three functional domains - hirudin fusion protein, which has the functions of targeted enrichment, long-acting circulation, local activation, precise anti- The characteristics of condensation ( figure 2 ).
[0058] 1. The inventors of the present invention obtained the sequence of the hirudin variant 2 (hirudin variant-2, HV2) polypeptide (UniProtKB / Swiss-Prot: P09945.1, sequence SEQ ID NO.13). R824 peptide, composed of 6 amino acids (PGDLSR, sequence SEQ ID NO.5), can specifically bind to PS with high affinity, IC 50 1.38×10 -9 M, as the target of "procoagulant platelets", the fusion protein containing R824 can be enriched by targeting "procoagulant platelets". The sequence of albumin...
Embodiment 2
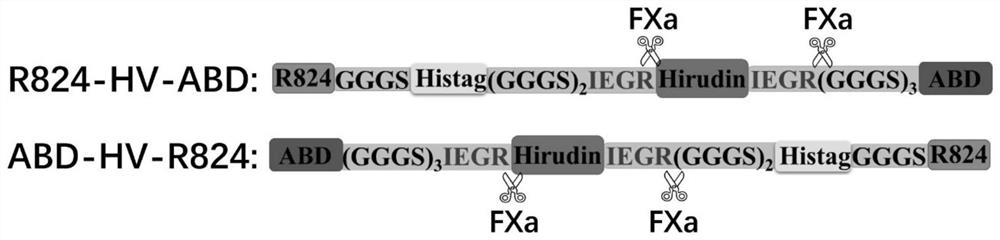
[0061] Embodiment 2, cDNA molecular synthesis of coding R824-HV-ABD and ABD-HV-R824 protein
[0062] 1. Design the gene sequence encoding hirudin fusion protein according to Escherichia coli preferred codon, the cDNA sequence encoding R824-HV-ABD is SEQ ID NO.3, and the cDNA sequence encoding ABD-HV-R824 is SEQ ID NO. 4.
[0063] 2. Sequence synthesis and expression vector construction
[0064] Entrusted Shanghai Jierui Bioengineering Co., Ltd. to synthesize the above two coding sequences, and construct and insert them into the pET30a vector (with NdeI and XhoI as insertion sites), and obtain the recombinant plasmid pET30a / R824-HV-ABD ( Figure 5 ) and pET30a / ABD-HV-R824 ( Figure 6 ), and then transformed Escherichia coli clone strain E.coli JM101. The recombinant plasmid was extracted and verified to be correct by DNA sequencing.
[0065] Transform the constructed expression plasmids pET30a / R824-HV-ABD and pET30a / ABD-HV-R824 into competent E.coli BL21(DE3) strains to obtai...
Embodiment 3
[0066] Embodiment 3, the expression acquisition of target protein
[0067] 1. According to the "Guidelines for Molecular Cloning", first inoculate the glycerol bacteria (constructed in Example 2) frozen at -20°C at a ratio of 1:1000 in the culture medium containing kan + (50 μg / mL) LB medium, shake overnight at 37°C.
[0068] 2. Inoculate the 1:100 ratio of the above-mentioned bacterial solution shaken overnight into the + (50μg / mL) in LB medium (1% NaCl, 1% tryptone, 0.5% yeast extract, autoclaved for later use), shake rapidly at 37°C for 4-5h, until OD 600nm ≈0.6 or so, the bacteria solution can be extracted every corresponding time for OD 600nm Determination, and drawn into OD curve to predict OD 600nm ≈0.6 time.
[0069] 3. Before the induction, the temperature was pre-cooled for 30 minutes. After cooling down to the induction temperature, IPTG (0.5M) was added to the final concentration for induction for 8 hours.
[0070] 4. Pre-weigh the centrifuge bottle, centrifug...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com