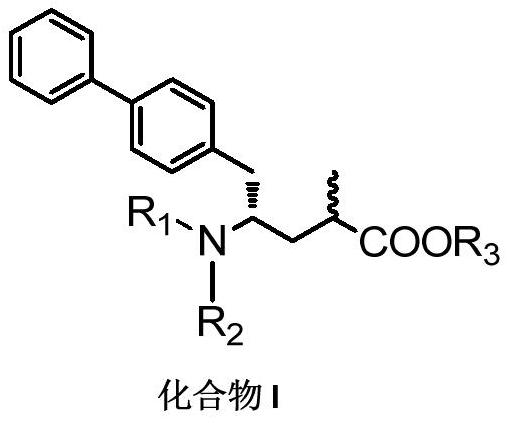
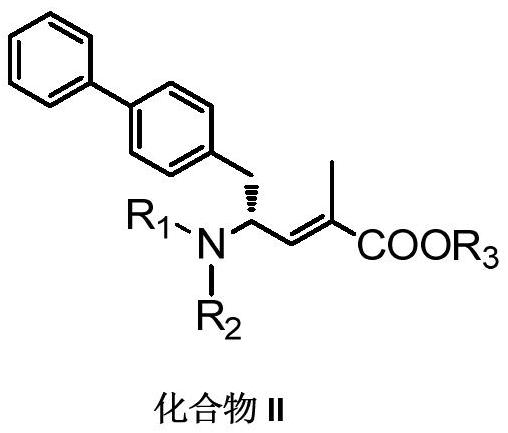
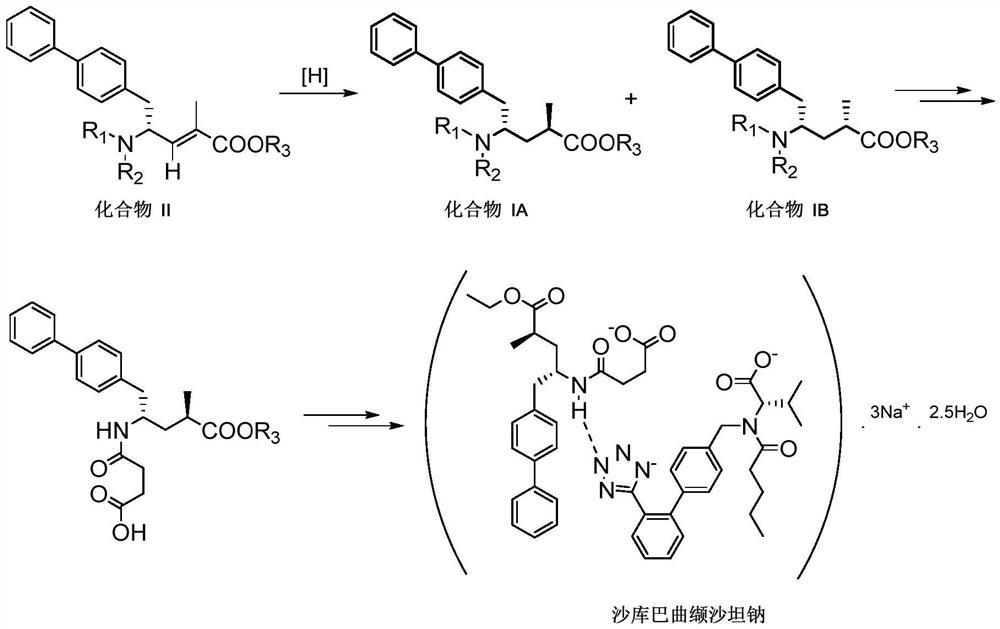
Method for preparing chiral biaryl substituted 4-amino-butyric acid and derivative thereof
An aryl and chiral ligand technology, which is applied in the field of preparing biaryl-substituted 4-amino-butyric acid or its derivatives, can solve the problem of insufficient stereoselectivity, unsuitable for industrial and commercial scale production, and expensive price. And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045]
[0046] Dissolve 2g of [Ru] catalyst (diiodo(p-cymene) ruthenium(II) dimer) and 1.3g of (R,R)-Me-Duphos in 20mL of toluene, react at 65-70℃ for one hour , prepared as a chiral catalyst. Add the above-mentioned chiral catalyst toluene solution into a clean hydrogenation kettle, and add SM1 (763g), triethylamine 202g, and 16L methanol solvent in sequence, and then replace the hydrogen gas (0.4-0.5MPa) three times with nitrogen in the closed hydrogenation kettle React under pressure at 55°C for 12 hours, take the reaction solution for liquid phase detection, the reaction SM1 conversion rate reaches 99.99%, product A:B=98.88:1.09, the reaction solution is filtered and concentrated, n-hexane and ethyl acetate (1 : 1) Pure product A can be obtained by beating; the yield is 96.1%, and the product is off-white solid.
[0047]1 HNMR (CD 3 OD, 400MHz): 1.15-1.17(d, J=8Hz, 3H), 1.22-1.36(d, 9H), 1.38-1.50(m, 1H), 1.85-1.93(m, 1H), 2.55-2.76(m , 3H), 3.82-3.85 (m, 1H), 7.25-...
Embodiment 2
[0050]
[0051] Dissolve 2g of [Ru] catalyst (diiodo(p-cymene) ruthenium(II) dimer) and 1.3g of (S,S)-Me-Duphos in 20mL of toluene, react at 65-70°C for one hour, prepared as a chiral catalyst. Add the above-mentioned chiral catalyst toluene solution into a clean hydrogenation kettle, and add SM1 (763g), triethylamine 202g, and 16L methanol solvent in sequence, and then replace the hydrogen gas (0.4-0.5MPa) three times with nitrogen in the closed hydrogenation kettle React under pressure at 55°C for 12 hours, take the reaction solution for liquid phase detection, the reaction SM1 conversion rate reaches 99.97%, product A:B=1.52:98.45, the reaction solution is filtered and concentrated, n-hexane and ethyl acetate (1 :1) Pure product B can be obtained by beating; the yield is 95.7%, and the product is off-white solid.
[0052] 1 HNMR (CH 3 OD, 400MHz): 1.13-1.15(d, J=8Hz, 3H), 1.23-1.35(d, 9H), 1.52-1.58(m, 1H), 1.78-1.88(m, 1H), 2.48-2.50(m , 1H), 2.74-2.78 (m, 2H), 3.82...
Embodiment 3
[0055]
[0056] Dissolve 1.3g of [Ru] catalyst (dichloro(p-cymene) ruthenium(II) dimer) and 1.3g of (R,R)-Me-Duphos in 20mL of toluene, react at 65-70℃ for one hour , prepared as a chiral catalyst. Add the above-mentioned chiral catalyst toluene solution into a clean hydrogenation kettle, and add SM1 (763g), triethylamine 202g, and 16L methanol solvent in sequence, and then replace the hydrogen gas (0.4-0.5MPa) three times with nitrogen in the closed hydrogenation kettle React under pressure at 55°C for 12 hours, take the reaction solution for liquid phase detection, the reaction SM1 conversion rate reaches 95.8%, product A:B=93.9:1.8, the reaction solution is filtered and concentrated, n-hexane and ethyl acetate (1 : 1) Pure product A can be obtained by beating; the yield is 90.5%, and the product is off-white solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com