Zearalenone lactone hydrolase mutant S162P with improved thermal stability and application thereof
A technology of giaralenone lactone and hydrolase, applied in the field of enzyme engineering, can solve the problems of secondary pollution, low removal efficiency, unfavorable environmental protection, animal and plant health, etc., and achieve the effect of improving thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: Preparation of mutant enzyme S162P
[0042] Construction of pET-22b(+)-S162P plasmid:
[0043] Construction of recombinant plasmid pET22b-Glro containing wild-type Glro: According to the gene encoding the ZEN lactonohydrolase of GliocladiumroseumMA918 (GenBank: KR363960.1), the gene fragment Glro of ZEN lactonohydrolase was synthesized and connected to the vector pET-22b (+) was cut between Nde I and Xho I to obtain recombinant plasmid pET22b(+)-Glro.
[0044] Using the pET-22b(+)-Glro plasmid as a template, through PCR 1 and PCR 2, the S162P site-directed mutation was introduced, and the sequencing verification results showed that there was no random mutation except the required mutation site, indicating that the mutant plasmid pET-22b( +)-S162P built successfully.
[0045] Mutation primers are as follows: (underlined are mutants)
[0046] Forward mutation primer: 5'-GTCTGGAGGC CCG GAGGCGTGGCAAGCCATG-3',
[0047] Reverse mutation primer: 5'-CCACGCCTC ...
Embodiment 2
[0055] Embodiment 2: the expression purification method of mutant enzyme
[0056] The mutant plasmid pET-22b(+)-S162P verified by sequencing was transformed into large E.coli BL21(DE3) cells, and the positive transformants were picked and cultured overnight in LB medium at 37°C and 200rpm, and then cultured in LB Base cultured at 37°C for 3h-4h to OD value of 0.6-0.8, cooled to 28°C, added IPTG so that its final concentration was 0.6mM, and induced for 6h.
[0057] The fermentation broth was centrifuged at 4°C and 10000 rpm for 20 min, and the cells were collected. Add 20mL of buffer solution (50mM Tris, 200mMNaCl, HCl to adjust the pH to 8.5) to fully resuspend the bacteria, then place the centrifuge tube in an ice bath and put it into an ultrasonic cell disruptor. The conditions for ultrasonic disruption are: working time 1 s, The stop time is 2s, a total of 18min. The obtained crushed solution was subjected to low-temperature high-speed centrifugation at 4°C and 10,000 rp...
Embodiment 3
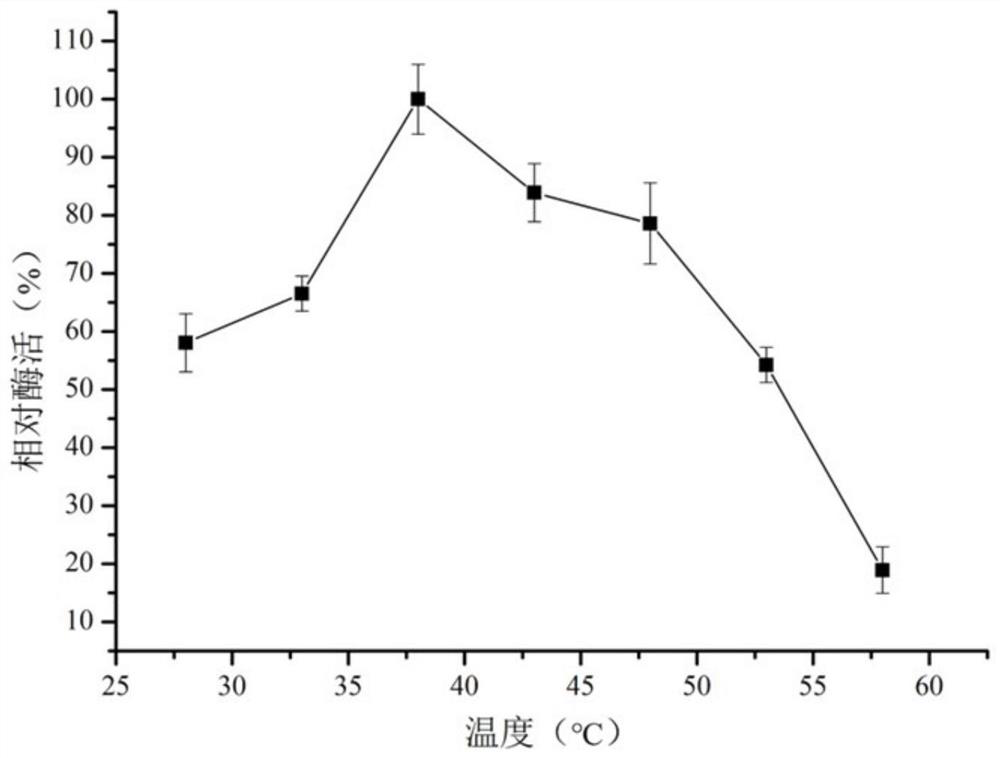
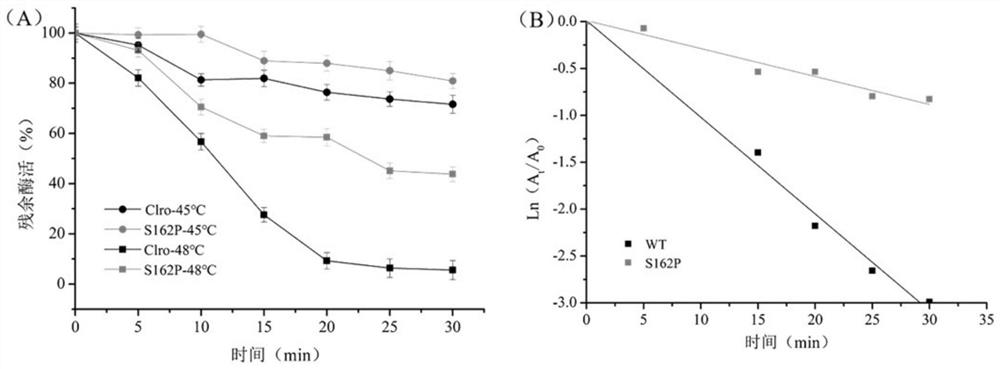
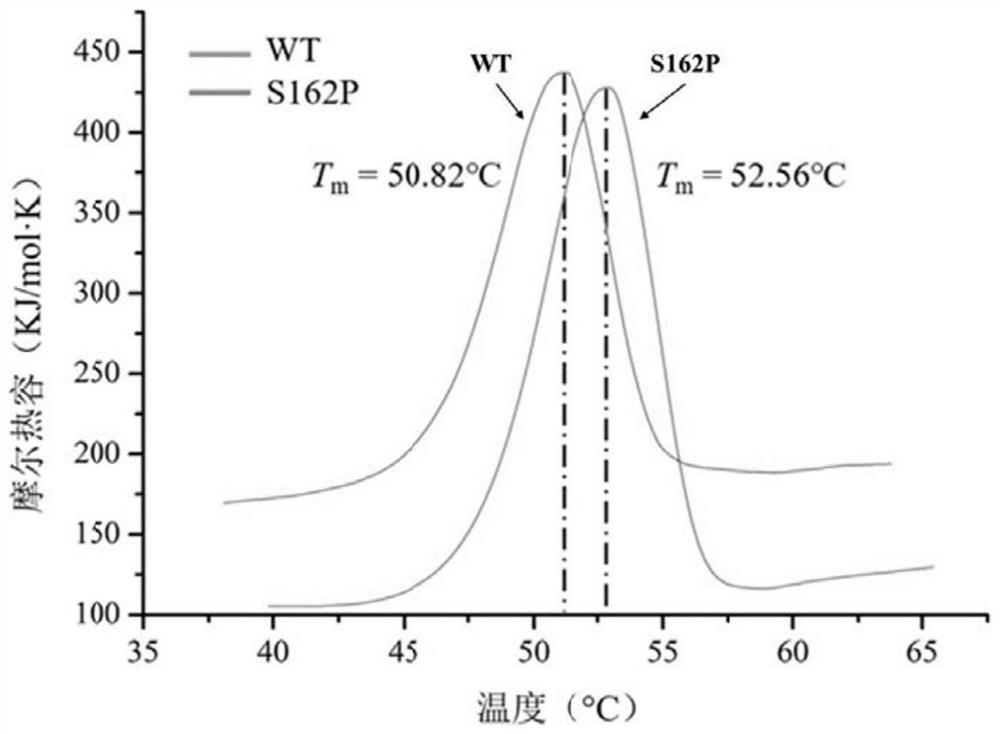
[0059] Example 3: Thermostability determination of mutant enzyme S162P
[0060] The thermal stability changes of the enzymes before and after the mutation were compared, wherein the wild enzyme refers to the ZEN lactonohydrolase (GenBank: ALI16790.1) derived from Gliocladium roseum MA918, and the mutant enzyme refers to the mutant enzyme S162P prepared in Examples 1 and 2.
[0061] (1) Determination of ZEN lactone hydrolase enzyme activity:
[0062] Standard reaction system: 5 μL of ZEN in methanol solution (4 mg / mL ZEN), 5 μL of enzyme (0.5 mg / mL) and 240 μL of phosphate buffer (50 mM, pH 7.0), reacted at 38°C for 10 min, added 50 μL of 1N hydrochloric acid and 300 μL of methanol was used to stop the reaction. 1U of total enzyme activity is defined as the amount of enzyme required to consume 1 μg of substrate per minute at pH 7.0 and 38°C.
[0063] The synthetic amount of ZEN was detected by HPLC, and the specific enzyme activity of mutant S162P was calculated to be 320U / mg...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com