TiO2 nanowire array substrate and preparation method and application thereof
A nanowire array and substrate technology, applied in biochemical equipment and methods, cell dissociation methods, tumor/cancer cells, etc., can solve the problems of impaired cell activity, time-consuming, expensive equipment, etc., and achieve high efficiency and specificity. The effect of capturing, increasing surface area, and improving capture efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
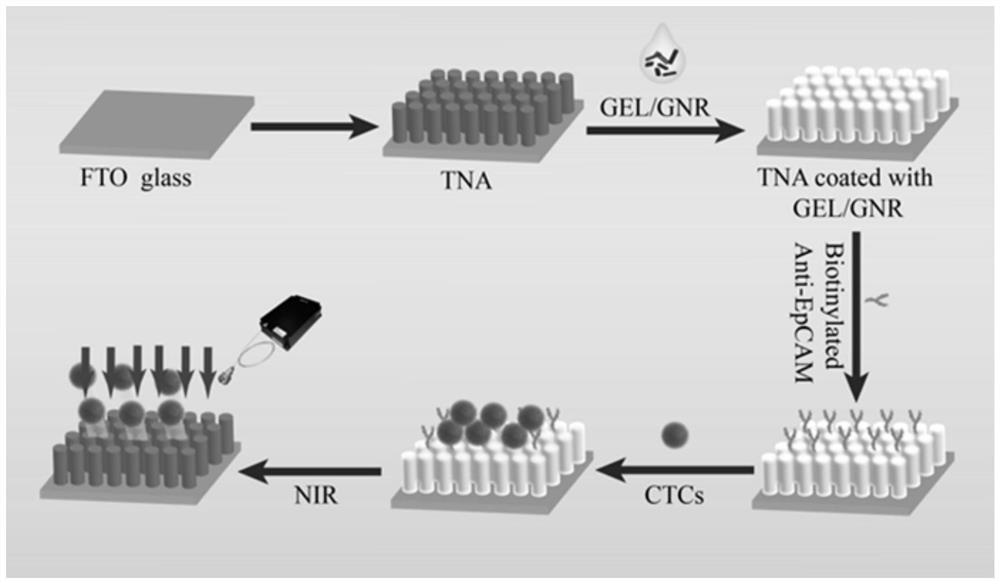
[0054] Preparation of antibody-modified TiO 2 Nanowire Array Substrate.
[0055] 1. Preparation of TiO 2 array of nanowires.
[0056] (1) Synthesis of TiO by hydrothermal method on the substrate 2 The nanowire array, the specific steps are as follows: (1) ultrasonically clean the FTO glass with acetone, absolute ethanol, and triple-distilled water for 15 minutes in sequence;
[0057] (2) Place the cleaned FTO glass in an oven at 65°C to dry;
[0058] (3) 30ml of concentrated hydrochloric acid with a concentration of 12mol / L, 30ml of deionized water and 1ml of tetrabutyl titanate were poured into the Erlenmeyer flask in sequence, and the reaction solution was obtained after mixing;
[0059] (4) Place the FTO glass dried in step (2) in the reactor, then pour the reaction solution obtained in step (3), then place the reactor at a temperature of 155° C., and react for 8 hours, namely have to.
[0060] 2. Apply gelatin.
[0061] (1) Doping gold nanorods in gelatin, the conce...
experiment example 2
[0070] Cell capture experiments.
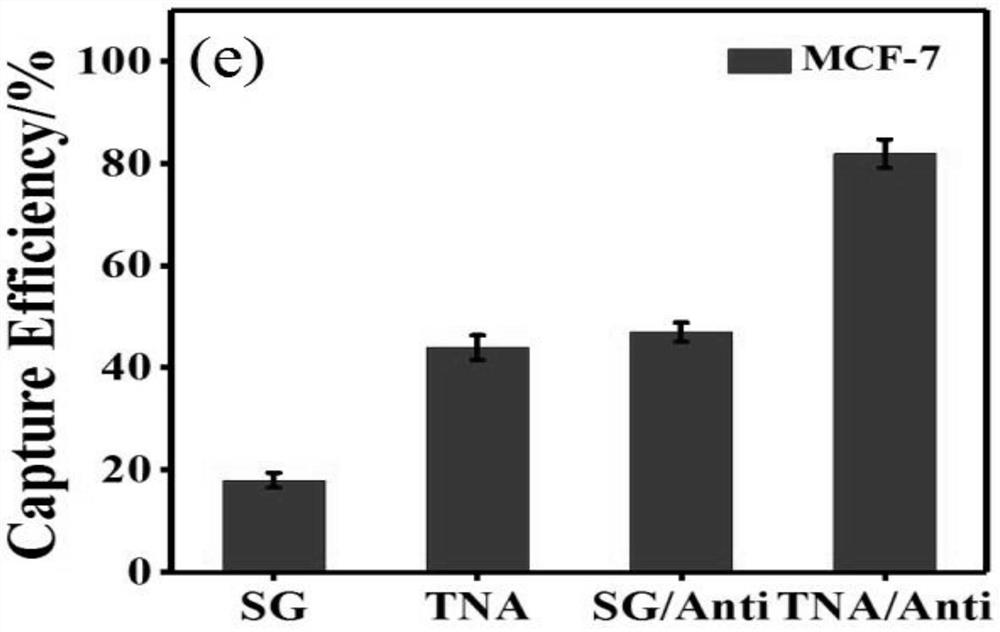
[0071] 1. Set smooth glass group (SG), smooth glass group modified with antibody (SG / Anti) and TNA group as experimental controls;
[0072] 2, the TiO obtained in embodiment 1 2 The nanowire array substrate and the above three groups of experimental control substrates, a total of four substrates were co-incubated with MCF-7 cells;
[0073] 3. Specific incubation conditions: the concentration of MCF-7 cells is 10 5 cell / ml, incubate at room temperature for 1 hour.
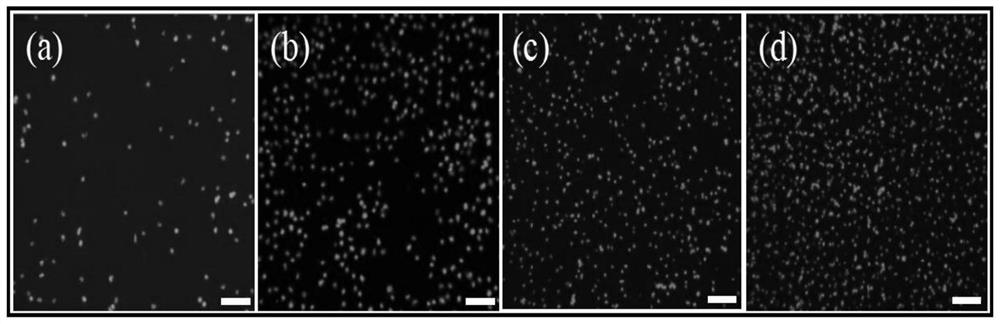
[0074] The result is as figure 2 , image 3 shown, where figure 2 a, b, c, d in image 3 Smooth glass group (SG), TiO of unmodified antibody in 2 The nanowire array substrate (TNA), the smooth glass group (SG / Anti) modified with antibodies, and the substrate (TNA / Anti) obtained in Example 1 represent the cell density distribution under the fluorescence field of view. It can be seen from the statistical diagram that due to The joint effect of the morphology and the antibod...
Embodiment 3
[0076] Specific capture cell experiments.
[0077] 1, with the TiO that embodiment 1 obtains 2 Nanowire array substrates were co-incubated with MCF-7, A549, Hela, and WBC;
[0078] 2. The specific incubation conditions are: the concentrations of MCF-7, A549, Hela, and WBC cells are all 10 5 cell / ml, respectively and the TiO obtained in Example 1 2 The nanowire array substrate was incubated at room temperature for 1 hour.
[0079] The result is as Figure 4 As shown, it was found that the substrate modified with Anti-EpCAM antibody in Example 1 efficiently captured MCF-7 and A549 cells specifically expressing EpCAM antigen, but less captured Hela and WBC cells that did not express EpCAM antigen. This shows that the substrate has the ability to specifically recognize the captured cells.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com