Phenothiazine compound as well as preparation method and application thereof
A technology of phenothiazines and compounds, applied in the field of phenothiazines and their preparation, to achieve the effects of high reaction yield, mild reaction conditions and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
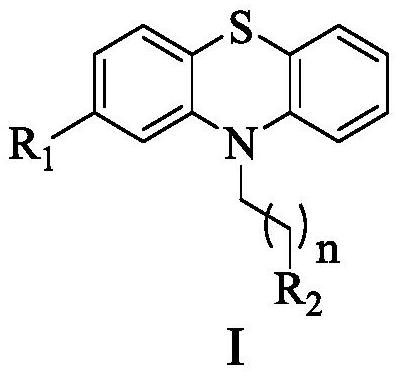
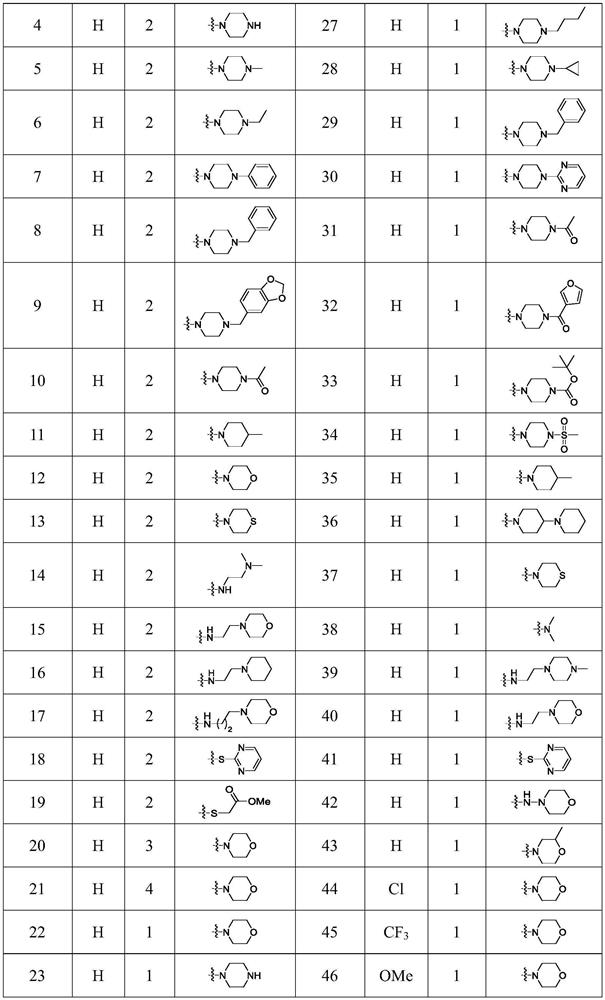
[0039] The preparation process of compound 4 to compound 21 is as follows:
[0040]
Embodiment 1
[0042] Preparation of Compound 4: n=2,
[0043] (1) Dissolve 1a (1.99g, 10mmol) in anhydrous N,N-dimethylformamide (20mL), then add 1-bromo-3-chloropropane (1.89g, 12mmol) and sodium hydride in sequence (0.48g, 12mmol, 60% purity), reacted at room temperature, and monitored the progress of the reaction by TLC. After the reaction is complete, remove N,N-dimethylformamide by rotary evaporation, dissolve the residue in ethyl acetate, wash with water and saturated brine in turn, dry the organic phase with anhydrous magnesium sulfate, filter, and remove the solvent by rotary evaporation Afterwards, compound 2b was obtained by column chromatography. The yield was 61.8%.
[0044] 1 H NMR (400MHz, CDCl 3 )δ7.17–7.15(m, 4H), 7.06(d, J=7.5Hz, 2H), 6.94(d, J=7.5Hz, 2H), 4.08(t, J=6.5Hz, 2H), 3.67( t,J=6.1Hz,2H),2.24–2.21(m,2H). 13 C NMR (100MHz, CDCl 3)δ145.06, 144.49, 133.18, 127.64, 127.30, 127.16, 125.70, 123.01, 122.73, 122.39, 115.57, 115.29, 43.92, 42.45, 29.65. HRMS (ESI)...
Embodiment 2
[0048] Preparation of Compound 5: n=2,
[0049] The difference between embodiment 2 and embodiment 1 is that the piperazine in step (2) is replaced by N-methylpiperazine, the alkaline substance in step (4) is pyridine, sodium hydroxide, and all the other are the same as embodiment 1 . Compound 5 was prepared with a yield of 81.4%.
[0050] 1 H NMR (400MHz, CDCl 3 )δ7.15–7.12(m,4H),6.92–6.87(m,4H),3.92(t,J=6.9Hz,2H),2.50–2.47(m,10H),2.28(s,3H),1.99 –1.92(m,2H). 13 C NMR (100MHz, CDCl 3 )δ145.21, 127.44, 127.21, 125.07, 122.42, 115.51, 55.59, 54.99, 53.06, 45.87, 45.28, 24.38. HRMS (ESI) calcd for C 20 h 26 N 3 S[M+H] + ,340.1842; found, 340.1833.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com