Synthesis method of impurity of C-glycoside derivatives
A synthesis method and technology of derivatives, applied in the direction of sugar derivatives, sugar derivatives, preparation of sugar derivatives, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
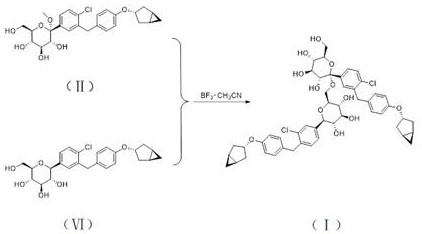
[0042] This example is used to illustrate the synthesis method of the compound shown in formula (I), including the following steps:
[0043] (1) Synthesis of intermediate 1
[0044] The compound represented by formula (II) (50 g, 101.8 mmol) was dissolved in ethyl acetate (500 mL), and TEA (61.81 g, 610.8 mmol) and DMAP (0.12 g, 1 mmol) were added. Acetic anhydride (51.96 g, 509 mmol) was added dropwise at a temperature of 0-10°C. After dropping, the temperature was raised to 25°C to react for 4 hours. Cool down to 0 °C and add aqueous sodium bicarbonate solution (200 mL) dropwise to quench the reaction. Adjust the pH to 5~6 with 1N dilute hydrochloric acid, and separate the liquids. The organic phase was backwashed with saturated brine (200 mL), dried over anhydrous sodium sulfate, and spin-dried. Add acetonitrile (1 L*2) to dissolve the remaining oil, replace it by distillation twice, and obtain intermediate 1 (68.43 g) represented by formula (Ⅲ) after distillation.
[...
Embodiment 2
[0049] Example 2: Confirm the structure of the compound prepared in Example 1
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com