Lidocaine hydrochloride gel and preparation method thereof
A technology of lidocaine hydrochloride and gel, which is applied in the direction of pharmaceutical formulations, medical preparations with no active ingredients, and medical preparations containing active ingredients. Weakness and other problems, to achieve the effect of blocking heat conduction, good transdermal effect, and reducing pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
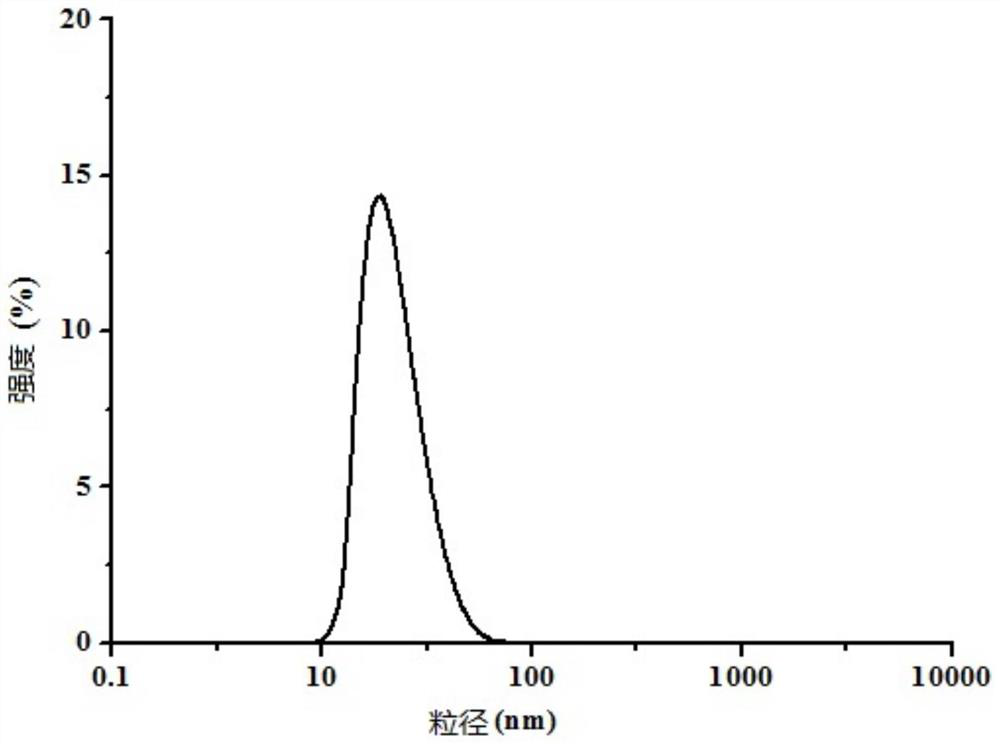
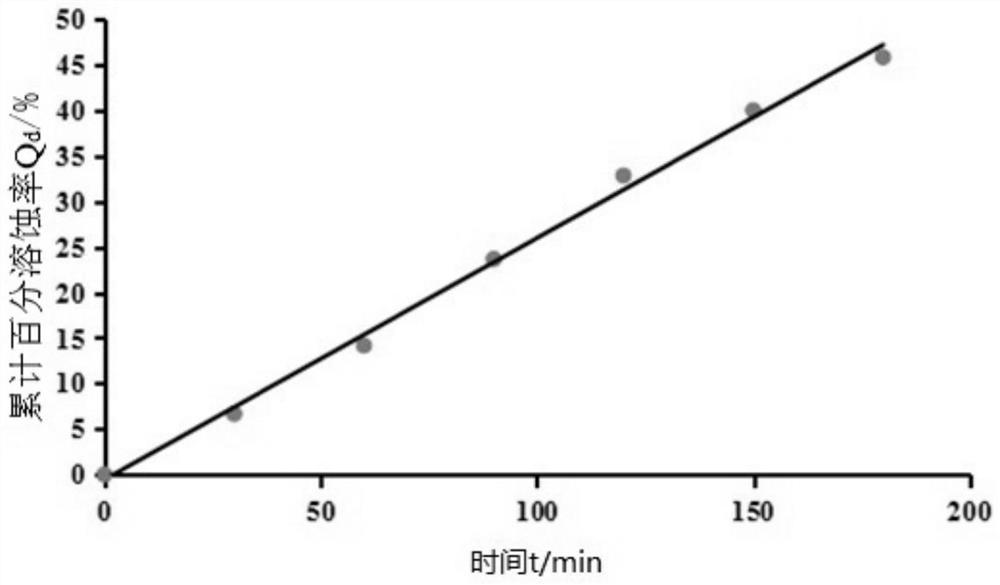
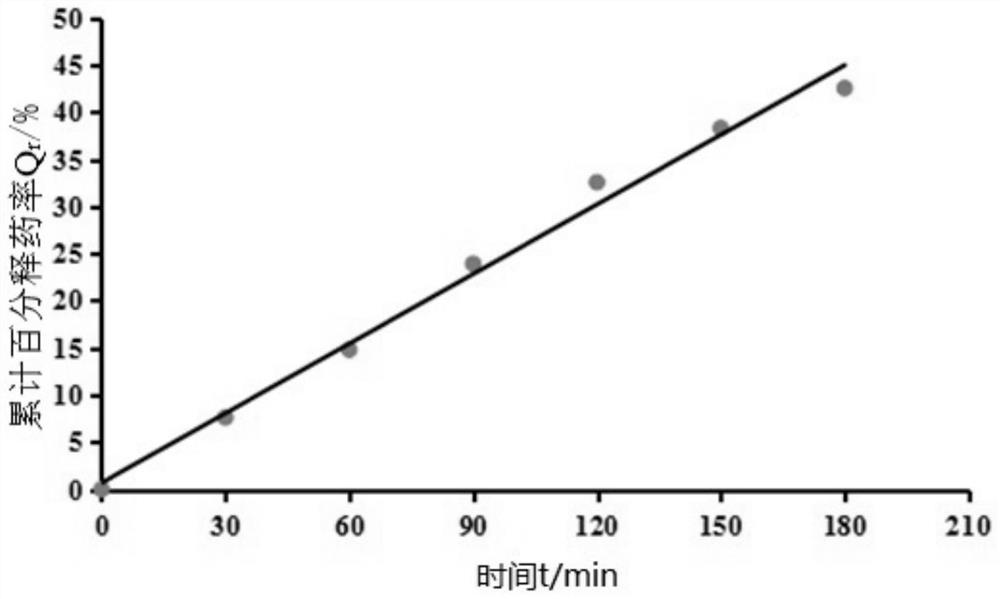
[0039] This embodiment provides a lidocaine hydrochloride gel, which comprises the following components in terms of mass percent: Poloxamer 407: 12%, Poloxamer 188: 0.3% and nanoemulsion: 87.7%, wherein, The above-mentioned nanoemulsion includes the following components based on the total amount of each component as 100%: ethyl oleate: 3%, Tween 80: 20%, absolute ethanol: 7%, deionized water: 68% and lidocaine hydrochloride Caine: 2%.
[0040] The preparation method of above-mentioned lidocaine hydrochloride gel comprises the following steps:
[0041] Step 1, taking each component according to the above-mentioned raw material ratio;
[0042] Step 2, adding the above-mentioned lidocaine hydrochloride to ethyl oleate, Tween 80 and dehydrated ethanol to dissolve to obtain an emulsion; Add dropwise to the emulsion at 25°C;
[0043] Step 3: Mix the above-mentioned poloxamer 407 and poloxamer 188 to obtain a gel matrix, add the above-mentioned gel matrix to the above-mentioned na...
Embodiment 2
[0045] This embodiment provides a lidocaine hydrochloride gel, which comprises the following components in terms of mass percent: Poloxamer 407: 13%, Poloxamer 188: 0.1% and nanoemulsion: 86.9%, wherein, The above-mentioned nanoemulsion includes the following components based on the total amount of each component as 100%: oleic acid: 2%, Tween 20: 21%, absolute ethanol: 5%, deionized water: 70% and lidocaine hydrochloride :2%.
[0046] The preparation method of above-mentioned lidocaine hydrochloride gel comprises the following steps:
[0047]Step 1, taking each component according to the above-mentioned raw material ratio;
[0048] Step 2, adding the above-mentioned lidocaine hydrochloride to oleic acid, Tween 20 and absolute ethanol to dissolve to obtain an emulsion; the deionized water is dripped at a rate of 4mL / min under the condition that the stirring speed is 13r / s Add it to the emulsion at 30°C to obtain nanoemulsion;
[0049] Step 3: Mix the above-mentioned poloxam...
Embodiment 3
[0051] This embodiment provides a lidocaine hydrochloride gel, which comprises the following components in terms of mass percent: Poloxamer 407: 11%, Poloxamer 188: 0.5% and nanoemulsion: 88.5%, wherein, The above-mentioned nanoemulsion includes the following components based on the total amount of each component as 100%: isopropyl myristate: 5%, laurate: 23%, glycerin cocoate: 10%, deionized water: 60% % and lidocaine hydrochloride: 2%.
[0052] The preparation method of above-mentioned lidocaine hydrochloride gel comprises the following steps:
[0053] Step 1, taking each component according to the above-mentioned raw material ratio;
[0054] Step 2, adding the above-mentioned lidocaine hydrochloride to isopropyl myristate, laurate and glyceryl cocoate to dissolve to obtain an emulsion; Add dropwise to the emulsion at 28°C at a rate of mL / min;
[0055] Step 3: Mix the above-mentioned poloxamer 407 and poloxamer 188 to obtain a gel matrix, add the above-mentioned gel matri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com