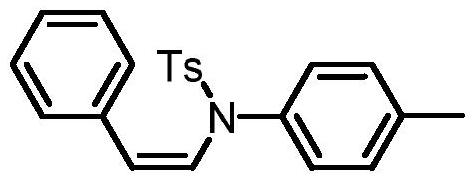
Preparation method of cis-N-styryl amide derivative
A technology of styrylamide and phenylethynylamide, applied in the field of preparation of cis-N-styrylamide derivatives, achieving high yield, green post-treatment, and wide substrate range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation of (Z)-N-Styryl-N-(p-tolyl)methanesulfonyl
[0025]
[0026] Add 0.1mmol of N-(phenylethynyl)-N-(p-tolyl)methanesulfonamide, 0.2mmol of p-toluenesulfonyl hydrazide, 0.15mmol of sodium carbonate and 1mL of tert-butanol into a 15mL reaction tube, and place In an oil bath at 80°C, react for 12 hours; cool to room temperature, dilute the reaction solution with ethyl acetate, wash three times with water, and wash the organic phase successively with anhydrous Na 2 SO 4 Drying, filtration, concentration and purification by TLC gave 24.1 mg of the target product with a yield of 84%. The NMR and high-resolution mass spectrometry characterizations of the target product obtained in this embodiment are as follows: 1 H NMR (500MHz, Chloroform-d) δ=7.14–7.09(m,4H),7.04–6.97(m,3H),6.91–6.86(m,2H),6.59(d,J=9.0Hz,1H), 6.04(d, J=9.0Hz, 1H), 2.83(s, 3H), 2.14(s, 3H); 13 C NMR (126MHz, Chloroform-d) δ = 137.1, 136.4, 133.8, 129.4, 128.9, 127.6, 127.1, 126.7, 126.3, 121.7...
Embodiment 2
[0028] Preparation of (Z)-4-methyl-N-styryl-N-(p-tolyl)benzenesulfonamide
[0029]
[0030] Add 0.1 mmol of N-(phenylethynyl)-N-(p-tolyl)-p-toluenesulfonamide, 0.2 mmol of p-toluenesulfonyl hydrazide, 0.15 mmol of sodium carbonate and 1 mL of tert-butanol into a 15 mL reaction tube, Placed in an oil bath at 80°C, reacted for 12 hours; cooled to room temperature, diluted the reaction solution with ethyl acetate, washed three times with water, and washed the organic phase successively with anhydrous Na 2 SO 4 Drying, filtration, concentration and purification by TLC gave 25.2 mg of the target product with a yield of 67%. The NMR and high-resolution mass spectrometry characterizations of the target product obtained in this embodiment are as follows: 1 H NMR (500MHz, Chloroform-d) δ=7.48–7.44(m,2H),7.22(td,J=5.9,2.8,4H),7.09–7.02(m,3H),6.91–6.84(m,4H) ,6.53(d,J=9.1,1H),6.03(d,J=9.0,1H),2.40(s,3H),2.18(s,3H); 13 C NMR (126MHz, Chloroform-d) δ = 143.9, 136.9 (d, J = 9.1), 134...
Embodiment 3
[0032] Preparation of (Z)-N-Benzyl-N-Styryl Methanesulfonamide
[0033]
[0034] Add 0.1mmol of N-benzyl-N-(phenylethynyl)methanesulfonamide, 0.2mmol of p-toluenesulfonyl hydrazide, 0.15mmol of sodium carbonate and 1mL of tert-butanol into a 15mL reaction tube, and place at 80°C in an oil bath, reacted for 12h; cooled to room temperature, the reaction solution was diluted with ethyl acetate, washed three times with water, and the organic phase was successively washed with anhydrous Na 2 SO 4 Drying, filtration, concentration and purification by TLC gave 19.4 mg of the target product with a yield of 65%. The NMR and high-resolution mass spectrometry characterizations of the target product obtained in this embodiment are as follows: 1 H NMR (500MHz, Chloroform-d) δ=7.44–7.41(m,2H),7.39–7.30(m,3H),7.27–7.24(m,3H),7.18(dd,J=7.3,2.2,2H) ,6.20(d,J=8.7,1H),6.14(d,J=8.7,1H),4.53(s,2H),2.81(s,3H); 13 C NMR (126MHz, Chloroform-d) δ = 135.7, 134.4, 128.9 (d, J = 2.8), 128.7, 128.4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com