Method for treating toxoplasmosis by using isoflavanoid compound glabridin
A technology of glabridin and toxoplasmosis, applied in the field of medicine, to achieve the effect of inhibiting invasion and proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The invention provides a method for treating toxoplasmosis by using the isoflavane compound glabridin, and the specific steps are as follows:
[0032] Step 1: Utilize isoflavane compound glabra to make medicines;
[0033] The isoflavane compound glabridin is extracted from licorice;
[0034] The medicine is composed of the following raw materials in parts by weight: 0.01-0.2 parts of isoflavane compound glabridin, 20-30 parts of disintegrating agent, 20-30 parts of filler, 2-6 parts of glidant, adhesive 2-8 parts, 1-3 parts of surfactant, and 1-3 parts of flavoring agent; the disintegrant is one of dry starch, sodium carboxymethyl starch, low-substituted cellulose, and cross-linked PVP. The filler is one of mannitol, lactose, microcrystalline cellulose; the glidant is one of talcum powder, micropowder silica gel, and the binder is starch slurry, methylcellulose, polydimensional One of ketones; the surfactant is one of sodium lauryl sulfate, stearic acid, and the corre...
Embodiment 2
[0043] The medicine is composed of the following raw materials in parts by weight: 0.2 parts of isoflavane compound glabridin, 30 parts of disintegrant, 30 parts of filler, 6 parts of glidant, 8 parts of binder, and 3 parts of surfactant and 3 parts of flavoring agent; the disintegrating agent is sodium starch glycolate, the filler is mannitol, the glidant micropowder silica gel, the binder is starch slurry, and the surfactant is sodium lauryl sulfate, and the flavoring agent is stevioside.
[0044] The specific steps of the preparation method of the medicine are as follows:
[0045] S1: sieve the raw material isoflavane compound glabridin, disintegrant, filler, glidant, binder, surfactant and flavoring agent separately for later use;
[0046] S2: uniformly mixing the isoflavane compound glabridin, a disintegrant, and a filler in a mixer to prepare a medicinal powder;
[0047] S3: making wet granules in a wet granulator with binder, surfactant, flavoring agent and medicinal ...
Embodiment 3
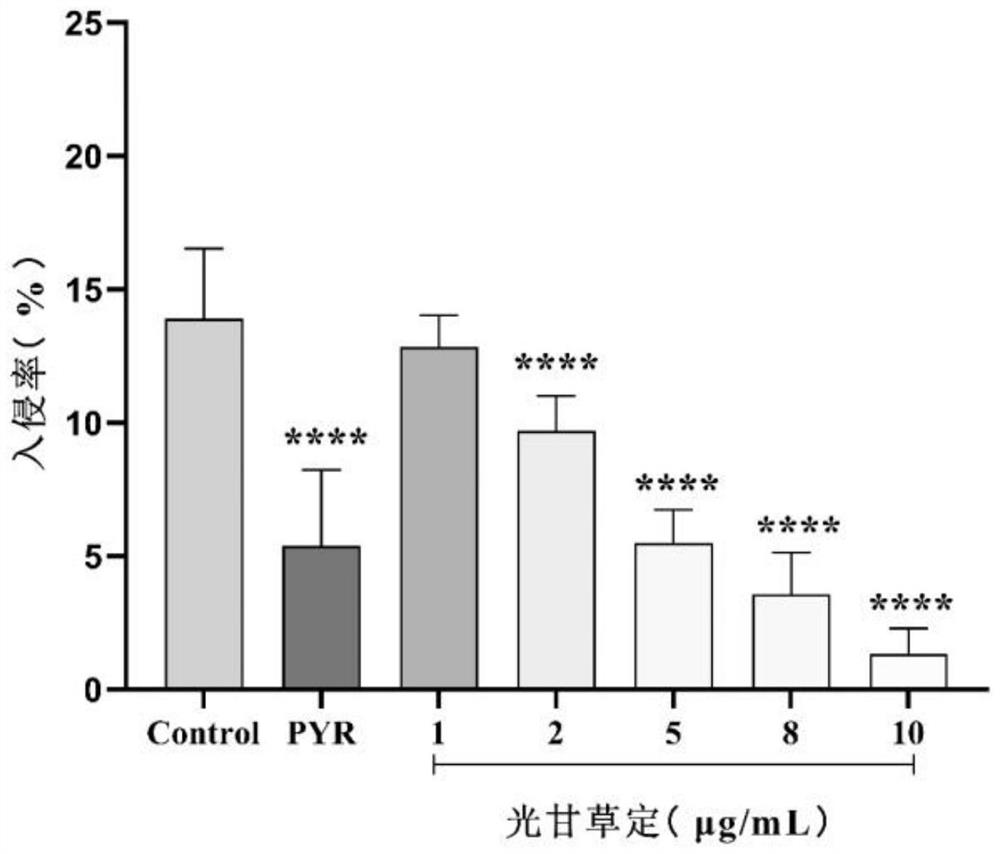
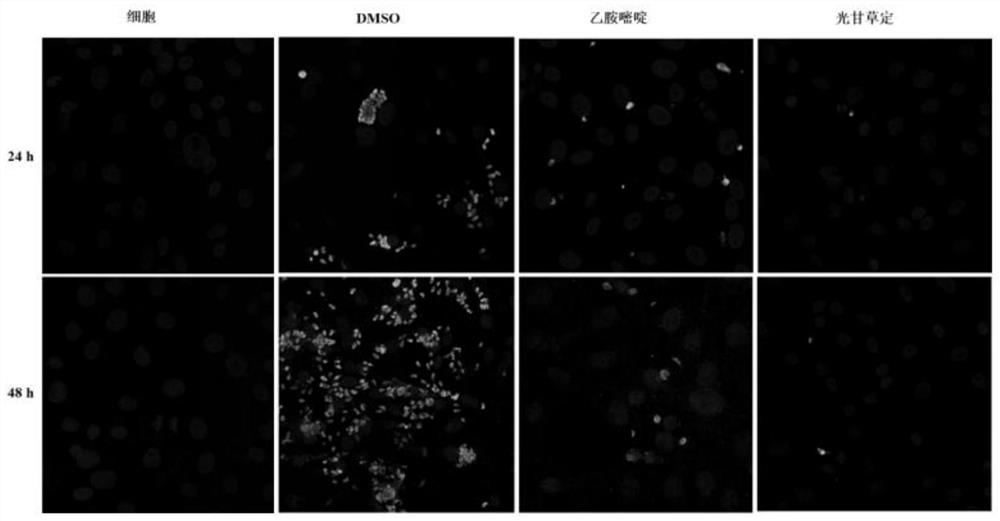
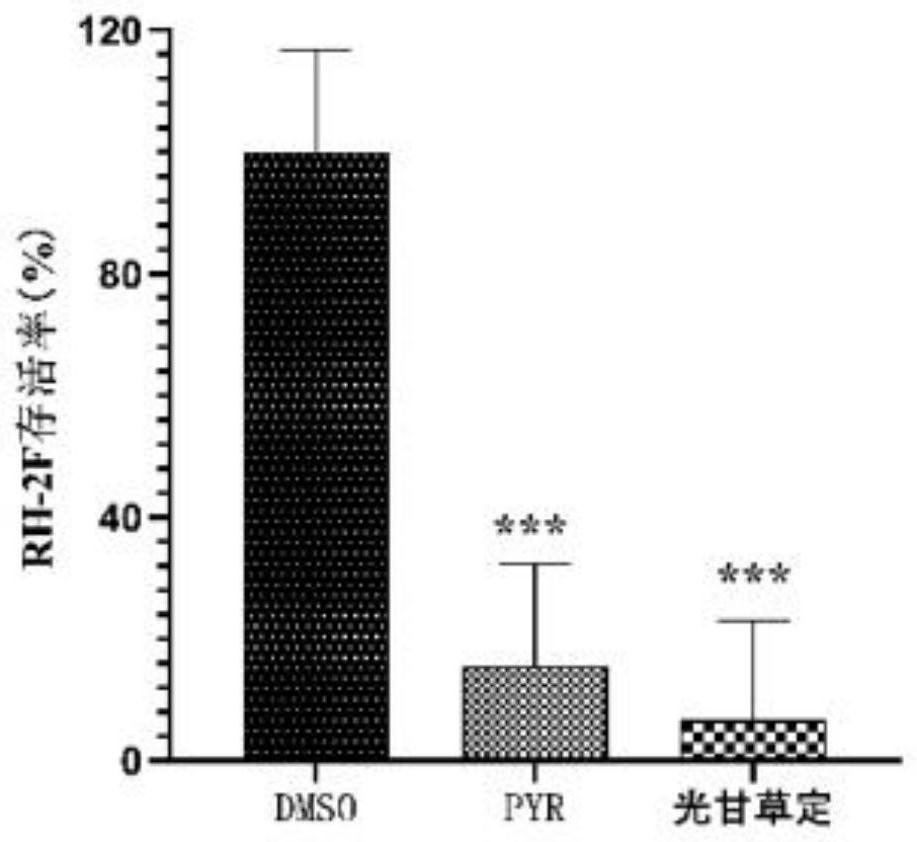
[0051] Determination of the anti-invasion effect of the medicine containing the isoflavane compound glabridin prepared in Example 2 on Toxoplasma gondii:
[0052] The drug was formulated as experimental samples containing the following concentrations of glabridin: 1 μg / mL, 2 μg / mL, 5 μg / mL, 8 μg / mL, 10 μg / mL.
[0053] Method 1: Determination of the anti-invasion effect of glabridin on Toxoplasma gondii RH by indirect immunofluorescence method:
[0054] Step: 1 × 10 containing each concentration of glabridin 5 RH tachyzoites / mL were inoculated in monolayer Vero cells after being treated for 1 h in the incubator, 0.25% DMSO was used as negative control group, and 10 μg / mL pyrimethamine (PYR) was used as positive control group. After 2 hours of invasion, try to wash away the non-invading tachyzoites with PBS. After fixation, antigen retrieval and blocking, the extracellular T. gondii was labeled with mouse anti-T. gondii monoclonal antibody, and the secondary antibody was goat an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com