Preparation method and anti-tumor application of conformation-locked melittin Anoplin derivative
A technology of conformation locking and melittin, which is applied in the preparation methods of peptides, antineoplastic drugs, chemical instruments and methods, etc., can solve the problems of limited clinical application, intolerance to protease hydrolysis, and the activity needs to be improved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
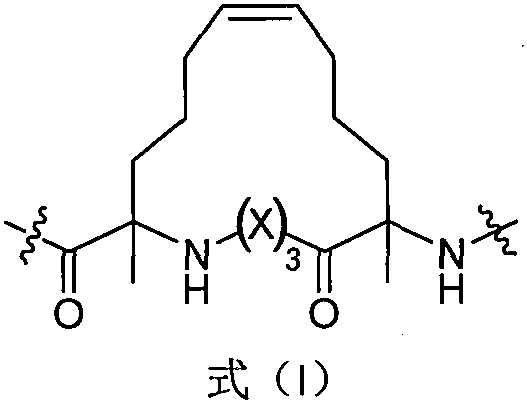
[0026] Example 1 Preparation of conformationally locked Anoplin derivatives Ano-1-6 of the present invention
[0027] Table 1 Amino acid sequences of the conformationally locked Anoplin derivatives Ano-1-6 of the present invention
[0028]
[0029] The conformationally locked Anoplin derivatives shown in Table 3 can be prepared by polypeptide solid-phase synthesis.
[0030] The preparation of the conformationally locked Anoplin derivative Ano-1 is described below as an example, and the preparation operations of the other conformationally locked Anoplin derivatives are the same.
[0031] synthetic route:
[0032]
[0033] Synthetic steps:
[0034] Weigh 667 mg of amino resin (degree of substitution 0.3 mmol / g) into a peptide synthesis tube, add 5 mL g of dichloromethane at room temperature to swell for 20 min, and then drain the solvent with a water pump. Add 5mL N,N-dimethylformamide to the above synthetic tube to wash the resin, add 7mL 20% piperidine / N,N-dimethylfor...
Embodiment 2
[0036] Example 2 The high-resolution mass spectrum and α-helical degree data of the conformationally locked Anoplin derivatives Ano-1-6 of the present invention
[0037] The high-resolution mass spectrum and α-helical degree data of the conformationally locked Anoplin derivatives Ano-1-6 of the present invention are shown in Table 2.
[0038] Table 2 High-resolution mass spectrum and α-helical degree data table of conformationally locked Anoplin derivatives Ano-1-6 of the present invention
[0039] It can be seen from the above table that the compounds of the present invention all increase the α-helix degree of Anoplin.
[0040]
Embodiment 3
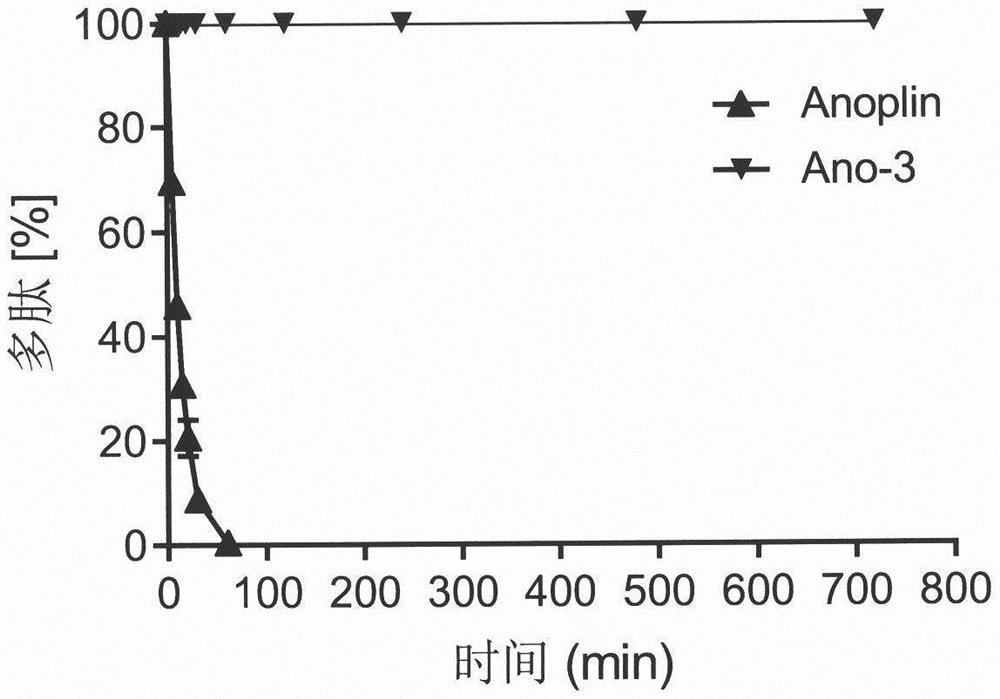
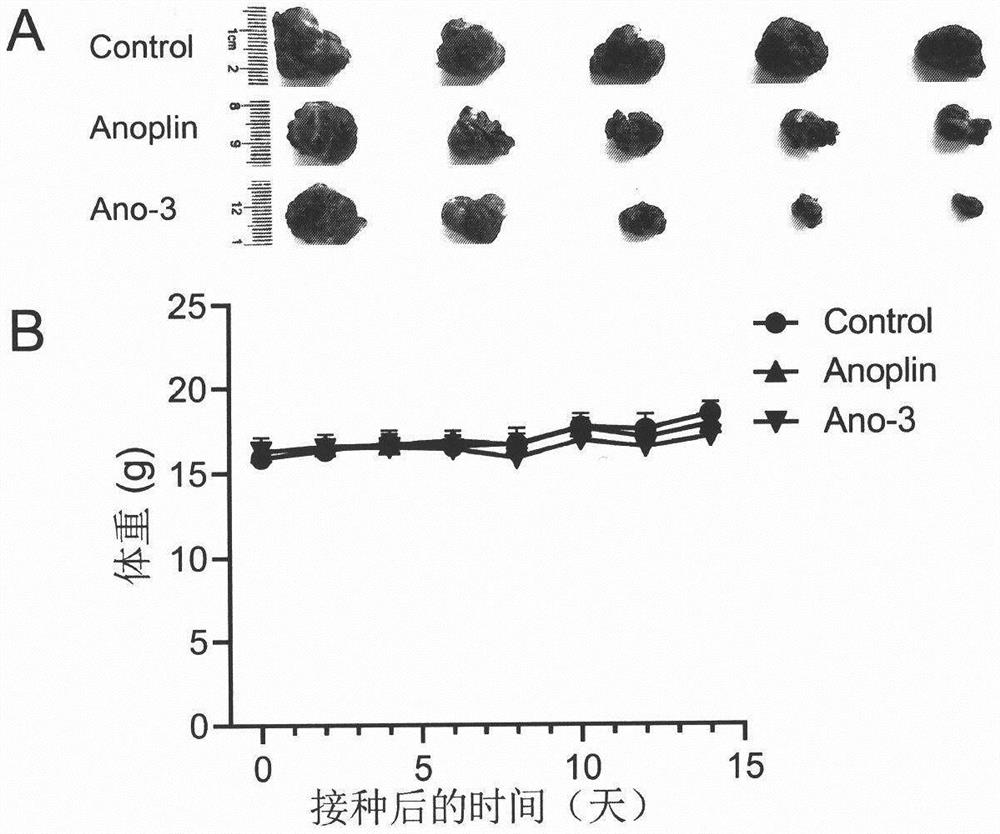
[0041]Example 3 In vitro anti-tumor experiment of conformationally locked Anoplin derivatives of the present invention
[0042] 3.1 Experimental method: CCK-8 cell proliferation toxicity detection kit was used to test the anti-proliferation activity of conformationally locked Anoplin derivatives on melanoma cell B16F10. Prepare 200 μL of B16F10 cell suspension in a 96-well plate, and place the culture plate at 37°C, 5% CO 2 The incubator was pre-incubated for 24 hours. Add 10 μL of different concentrations of conformationally locked Anoplin derivatives to the culture plate and incubate in the incubator for 24 hours. Add 10 μL of CCK-8 solution to each well. After the culture plate was incubated in the incubator for 2 hours, the absorbance of the 96-well plate at 450 nm was measured with a microplate reader. Calculate the inhibitory rate of the drug to B16F10 cells according to the following formula: inhibitory rate=[(Ac-As)] / [(Ac-Ab)]×100%, As: experimental well (culture me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com