Method for synthesizing isovanillin
A technology of isovanillin and ethyl vanillin, applied in the chemical field, can solve the problem of low reaction activity, and achieve the effects of optimizing reaction conditions, reducing costs, and being easy to recycle.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
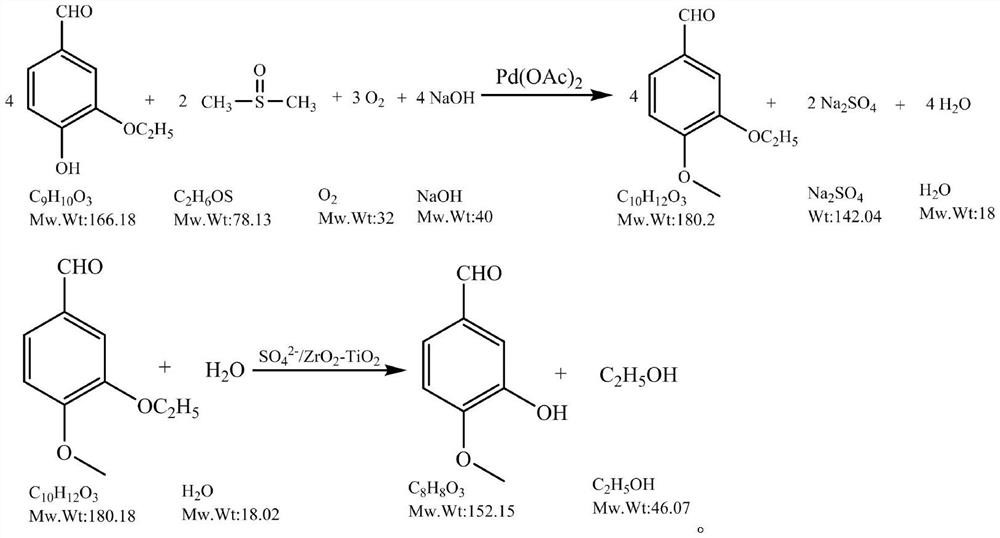
[0041] (1) Add 549g ethyl vanillin, 128g dimethyl sulfoxide, 5g Pd(OAc) in the reactor 2 , 264g sodium hydroxide solution (50%), oxygen replacement 3 times, the oxygen pressure is controlled at 0.2MPa, at 100°C, react for 6h, and finish the reaction. The reaction solution was filtered and separated to obtain an oil phase intermediate: 572.05 g of the crude product of ethoxy-4-methoxybenzaldehyde, based on the amount of ethyl vanillin, the yield of the intermediate was 97%, and the purity was 99.5%.
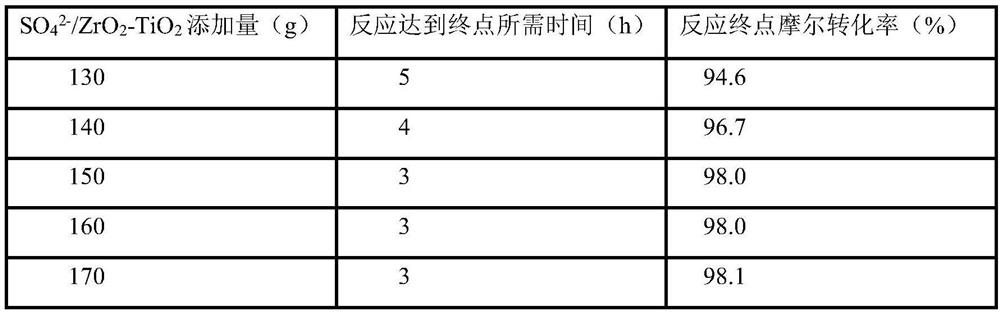
[0042] (2) Add 728g intermediate, 150g SO in the reactor 4 2- / ZrO 2 -TiO 2 Catalyst and 1500g of water were reacted at 65°C for 3h to end the reaction. Suction filter the reaction solution, dissolve the filter cake with 2000g ethanol, heat and stir at 60°C for 2h, and suction filter; cool the filtrate to 0°C, heat and stir for 2h, and suction filter to obtain 631.23g of wet product; the obtained solid is vacuum-dried to constant weight to obtain 3- Hydroxy-4-methoxybenzalde...
Embodiment 2
[0043] Embodiment 2 (amplification experiment)
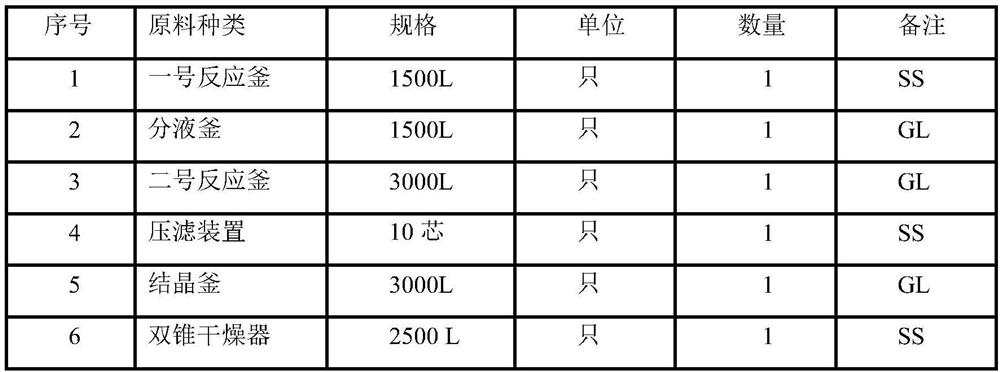
[0044] Major equipment
[0045]
[0046] (1) Add 549kg ethyl vanillin, 128kg dimethyl sulfoxide, 5kg Pd(OAc) in No. 1 reactor 2 , 264kg sodium hydroxide solution (50%), oxygen replacement 3 times, oxygen pressure is controlled at 0.2MPa, at 100 ℃, react 6h, finish reaction; Reaction liquid obtains the intermediate in oil phase through press filtration, liquid separation The crude product of ethoxy-4-methoxybenzaldehyde is 572.05g, based on the amount of ethyl vanillin.
[0047] (2) Add 728kg intermediate, 150kg SO in the reactor 4 2- / ZrO 2 -TiO 2 Catalyst, 1500kg water, react at 65°C for 3h, and finish the reaction. Pressure filter the reaction solution, dissolve the filter cake with 2000kg of ethanol, keep stirring at 60°C for 4 hours; press filter, transfer the filtrate to the crystallization kettle, cool down to 0°C, keep stirring for 4 hours; press filter to obtain 631.23kg of wet product, and transfer the filtrate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com