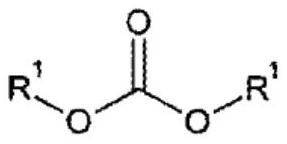
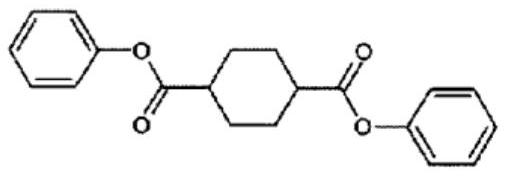
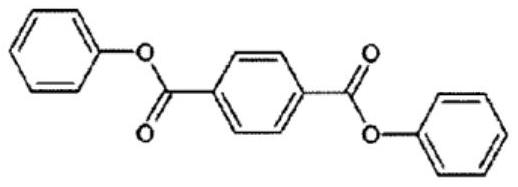
Polymerizable composition, and copolycarbonate ester and method for producing same
A polymer composition and copolycarbonate technology, which is applied in the field of polymer composition and copolycarbonate, can solve problems such as development restrictions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0175] Preparation example 1. Synthesis of bio-based TPA
[0176] Under nitrogen, 31.6g ethylenediamine (525mmol), 3.34g anhydrous FeCl 3 (145 mmol) and 0.16 g sodium (0.964 mmol) were mixed and heated at 50°C. 101 g of bio-based α-limonene (742 mmol; Sigma-Aldrich) was slowly added dropwise to the mixture, which was then heated to a temperature of 100° C. for 8 hours. Then, the reaction mixture was cooled to room temperature, diluted with 300 g of water and extracted twice with 400 g of dichloromethane (DCM). The extracted organic layer was dried with magnesium sulfate and evaporated using a rotary evaporator to obtain crude p-cymene (yield: 99%).
[0177] To a mixture of crude cymene and 400 g of water was added 288 g of 65% HNO 3 (2968 mmol), then allowed to react. The reaction mixture was refluxed with heating for 1 day, then cooled to room temperature and extracted with 530 g DCM. Then, the extracted organic layer was washed twice with water and evaporated using a ...
preparation example 2
[0179] Preparation example 2. Synthesis of bio-based DMT
[0180] To a mixture of 105 g of biobased TPA (632 mmol) obtained in Preparation 1 and 1650 g of methanol (6320 mmol) was added 3.1 g of concentrated H 2 SO 4 (31.6 mmol), which was refluxed with heating for 1 day. Thereafter, the solution was cooled to room temperature and the solid obtained after removal of the solvent was dissolved in 530 g of DCM. The solution was washed with water, and the organic layer was dried over magnesium sulfate and evaporated using a rotary evaporator to obtain a crude solid product. The solid product was washed with cold methanol and dried at 90 °C for 12 hours to obtain bio-based DMT (yield: 95%). Bio-based DMT has a bio-based carbon content of 83%.
preparation example 3
[0181] Preparation example 3. Synthesis of bio-based DMCD
[0182] A sheet-shaped molded catalyst in which 1% by weight of ruthenium (Ru) was supported on alumina was charged into a fixed-bed continuous reactor. The bio-based DMT obtained in Preparation Example 2 was supplied to the upper region of the reactor at a rate of 80 liters / hour together with hydrogen gas at a rate of 7 cm / sec, and was heated at 40 kgf / cm 2 The ring hydrogenation reaction is carried out under the reaction pressure. the reaction temperature is maintained at 140°C to 155°C in the upper zone of the reactor, at 135°C to 145°C in the middle zone of the reactor, and at 125°C to 135°C in the lower zone of the reactor, respectively, and The maximum temperature difference in the reactor is within 20 °C. After a reaction time of 5 hours to 10 hours, a crude liquid product is obtained from the lower region of the reactor. The liquid product was subjected to vacuum distillation to obtain bio-based DMCD. Bio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| transmittivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com