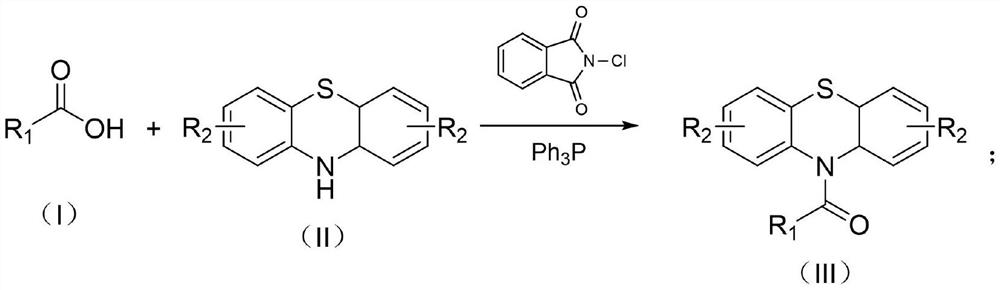
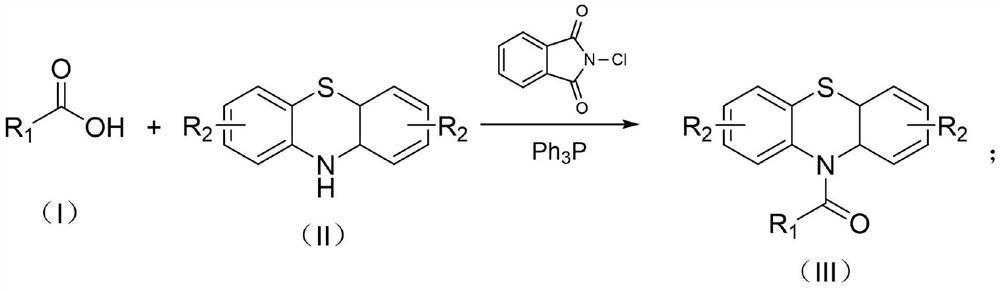
Preparation method of thermosensitive dye compound N-acyl phenothiazine
The technology of acyl phenothiazine and heat-sensitive dye is applied in the field of preparation of heat-sensitive dye compound N-acyl phenothiazine, which can solve the problems of incompletely clear action mechanism, exposed waste water, waste gas, easy generation of acid waste gas, etc. Stable yield, less environmental pollution and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0023] Example 1: Synthesis of N-(4-phenylbenzoyl) phenothiazine
[0024] Add N-chlorophthalimide (272mg, 1.5mmol), triphenylphosphine (393mg, 1.5mmol) and p-phenylbenzoic acid (198mg, 1.0mmol) respectively in a 25ml reaction tube equipped with a rotor, and then add 5mL solvent acetonitrile, after stirring for 5 minutes, add phenothiazine (199mg, 1.0mmol); stir at 25°C for 4 hours, the reaction solution was dissolved in 15ml ethyl acetate, saturated sodium bicarbonate solution (10ml) and brine (10ml) Each was washed once; the organic layer was dried over sodium sulfate, concentrated by rotary evaporation, and separated by column chromatography to obtain 362 mg of N-(4-phenylbenzoyl)phenothiazine with a yield of 95%.
example 2
[0025] Example 2: Synthesis of N-(4-chlorobenzoyl)-2-chlorophenothiazine
[0026] Add N-chlorophthalimide (272mg, 1.5mmol), triphenylphosphine (393mg, 1.5mmol) and p-chlorobenzoic acid (157mg, 1.0mmol) respectively in a 25ml reaction tube equipped with a rotor, and then add 5mL The solvent was acetonitrile, and after stirring for 5 minutes, 2-chlorophenothiazine (235 mg, 1.0 mmol) was added. After stirring at room temperature for 4 hours, the reaction solution was dissolved in 15 ml of ethyl acetate and washed once with saturated sodium bicarbonate solution (10 ml) and brine (10 ml). The organic layer was dried over sodium sulfate, concentrated by rotary evaporation, and separated by column chromatography to obtain 348 mg of N-(4-chlorobenzoyl)-2-chlorophenothiazine with a yield of 93%.
example 3
[0027] Example 3: Synthesis of N-(4-methylbenzoyl)phenothiazine
[0028] Add N-chlorophthalimide (272mg, 1.5mmol), triphenylphosphine (393mg, 1.5mmol) and p-toluic acid (136mg, 1.0mmol) respectively in a 25ml reaction tube equipped with a rotor, and then add 5 mL of solvent acetonitrile was stirred for 5 minutes and phenothiazine (199 mg, 1.0 mmol) was added. After stirring at room temperature for 8 hours, the reaction solution was dissolved in 15 ml of ethyl acetate and washed once with saturated sodium bicarbonate solution (10 ml) and brine (10 ml). The organic layer was dried over sodium sulfate, concentrated by rotary evaporation, and separated by column chromatography to obtain 293 mg of N-(4-methylbenzoyl)phenothiazine with a yield of 92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com