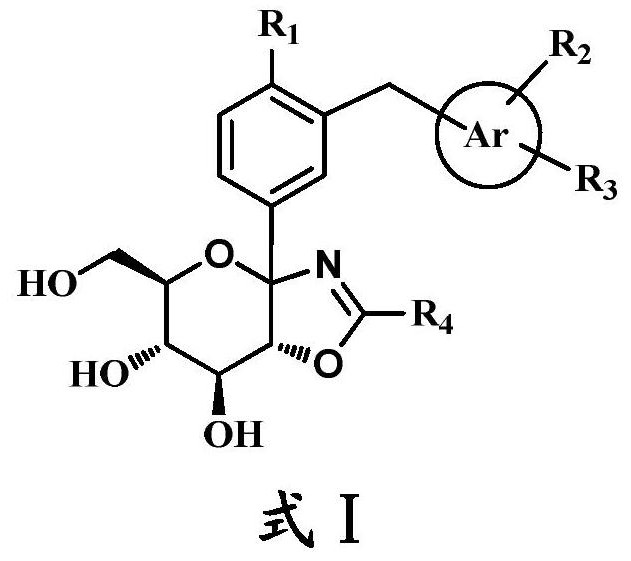
Bicyclic derivative of heterocyclic glucoside as well as preparation method and application of bicyclic derivative
A technology of heterocyclic groups and compounds, applied in the field of bicyclic derivatives of heterocyclic glucosides and their preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0195] Embodiment 1, the preparation of benzo [b] thiophene (5-bromo-2-chlorophenyl) methanol (Ⅴ-1)
[0196]
[0197] Dissolve benzothiophene (6.1g, 45.57mmol) in anhydrous tetrahydrofuran (50ml), under nitrogen protection, cool to -70°C with dry ice-acetone, slowly add 2.5M n-BuLi n-hexane solution (20ml, 50mmol) dropwise , so that the temperature is not higher than -60 ° C, and the drop is completed for 30 minutes, which is recorded as bottle A; 5-bromo-2-chlorobenzaldehyde (10.0g, 45.57mmol) is dissolved in anhydrous tetrahydrofuran (125ml), nitrogen protection, Cool down to -70°C and record as bottle B; transfer the solution in bottle A to bottle B and react for 4 hours. TLC monitoring showed that the reaction was complete. The low temperature bath was removed, and 30ml of purified water was added to quench. The tetrahydrofuran was removed by rotary evaporation, extracted with 3×100ml ethyl acetate, the organic phase was washed with 100ml saturated sodium chloride so...
Embodiment 2
[0200] Preparation of Example 2, 2-(5-bromo-2-chlorobenzyl)benzo[b]thiophene (Ⅲ-1)
[0201]
[0202] 6.0g (16.97mmol) of benzo[b]thiophene (5-bromo-2-chlorophenyl)methanol (V-1 (13.5ml, 84.85mmol) was slowly added dropwise with boron trifluoride ether (5.0ml, 40.73 mmol), so that the internal temperature is not higher than 10 ° C. Add, remove the ice bath, react for 4h. TLC detection, the reaction is complete. Add 2M potassium hydroxide solution to quench the reaction, stir. Extract with 3 × 20ml dichloromethane, organic The phases were washed successively with 3×25ml 2M potassium hydroxide solution, 3×40ml saturated sodium chloride solution, and dried over anhydrous sodium sulfate. The desiccant was filtered off and evaporated, and the residue was separated by silica gel column chromatography to obtain a white solid 3.8 g, yield 66.3%.
[0203] 1 H-NMR(400MHz,DMSO-d6,ppm)δ:7.87(1H,d),7.73-7.77(2H,m),7.53(1H,d),7.44(1H,d),7.27-7.35(2H, m),7.18(1H,m),4.36(1H,s);
[020...
Embodiment 3
[0205] Example 3, (3R, 4S, 5S, 6R)-2-(3-(benzo[b]thiophene-2-yl-methyl)-4-chlorophenyl)-6-(hydroxymethyl Preparation of -2-methoxy-5,6,7,7a-tetrahydro-2H-pyran-3,4,5-triol (Ⅱ-1)
[0206]
[0207] Dissolve 3.8g (11mmol) of 2-(5-bromo-2-chlorobenzyl)benzo[b]thiophene (Ⅲ-1) in anhydrous toluene (60ml), under nitrogen protection, add anhydrous tetrahydrofuran (20ml), Cool down to -70°C, slowly add 2.5M n-BuLi n-hexane solution (5.6ml, 14mmol) dropwise, so that the internal temperature is not higher than -60°C, and react for 30min after dropping, record as bottle A; put 2,3, 4,6-Tetra-O-trimethylsilyl-β-D-gluconolactone (5.8g, 12mmol) was dissolved in toluene (55ml), cooled to -70°C, recorded as bottle B; The solution was transferred to bottle B and reacted for 2h. TLC detects that the reaction is complete. Add methanesulfonic acid (6ml) and anhydrous methanol (110ml), remove the ice bath and nitrogen protection, and stir overnight at room temperature. As detected by TLC, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com