Preparation method of alminoprofen intermediate
A technology of intermediates and reaction solvents, applied in the field of organic chemical synthesis, can solve problems such as excessive waste, flammability of active nickel, and complicated post-treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
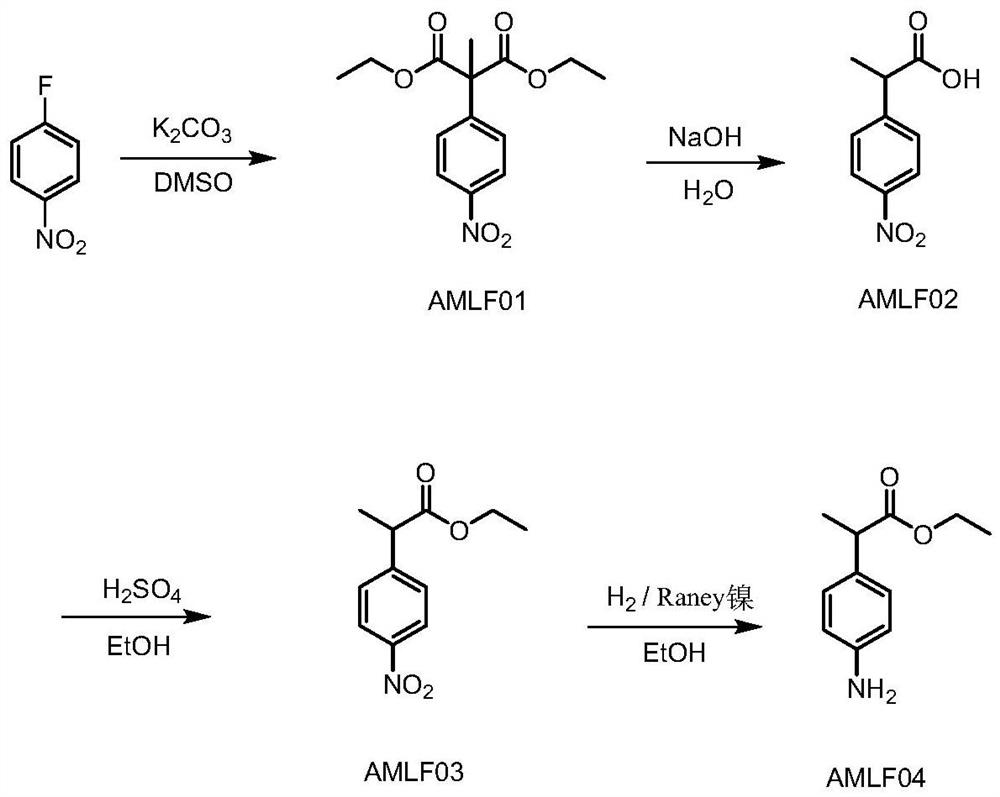
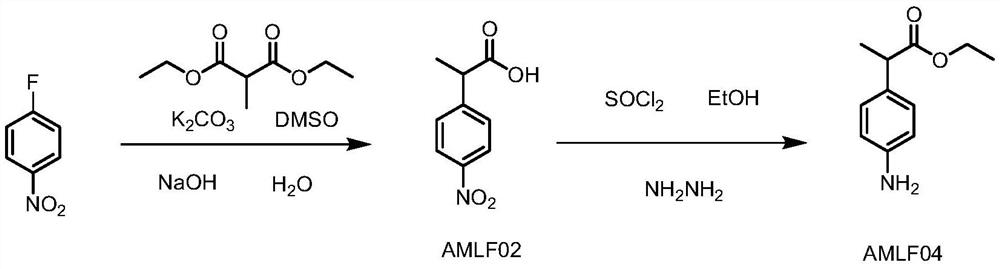
[0049] (1) Synthesis of AMLF02
[0050]
[0051] Add p-fluoronitrobenzene (50kg), DMSO (190.25kg), diethyl methylmalonate (70.4kg) into the reaction kettle, start stirring, add anhydrous powder potassium carbonate (73kg), and heat up to 70-80 °C, react for 15-17 hours. Cool down to 20-30°C, and add dropwise 30% sodium hydroxide solution (432kg) at a temperature of 20-50°C. After dropping, heat to 50-60°C for 1-2 hours. After standing for 1 h, the lower high saline phase was separated. Cool down to 20-30°C, add water (350kg), and adjust pH to 7-8 with concentrated hydrochloric acid. Dichloromethane (390 kg) was added to extract impurities twice. Control the temperature at 20-30°C and use concentrated hydrochloric acid to pH 2-3, add dichloromethane (455kg) and extract once. The organic phase was washed once by adding water (300kg), and concentrated to dryness under reduced pressure to obtain 128.6kg of AMLF02 with a crude product purity of 98.92%. The above-mentioned w...
Embodiment 2
[0056] (1) Synthesis of AMLF02
[0057]
[0058] Add p-fluoronitrobenzene (50kg), DMF (190.25kg), diethyl methylmalonate (70.4kg) into the reaction kettle, start stirring, add anhydrous sodium carbonate (56kg), and heat up to 70-80°C , react for 15-16 h. After the reaction is finished, the temperature is lowered to 20-30° C., and 30% potassium hydroxide solution (432 kg) is added dropwise at 20-50° C. under temperature control. After dropping, heat to 50-60°C and react for 1-2h. After standing for 1 h, the lower high saline phase was separated. Cool down to 20-30°C, add water (350kg), and adjust pH to 7-8 with concentrated hydrochloric acid. Impurities were extracted twice by adding ethyl acetate (390 kg). Control the temperature at 20-30°C and use concentrated hydrochloric acid to pH 2-3, add ethyl acetate (455kg) and extract once. The organic phase was washed once by adding water (300 kg), concentrated to dryness under reduced pressure to obtain 127.5 kg of AMLF02, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com