Method for regenerating functional retinal ganglion cells by using transcription factor
A retinal ganglion and transcription factor technology, applied in the field of biomedicine, to achieve the effect of easy promotion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
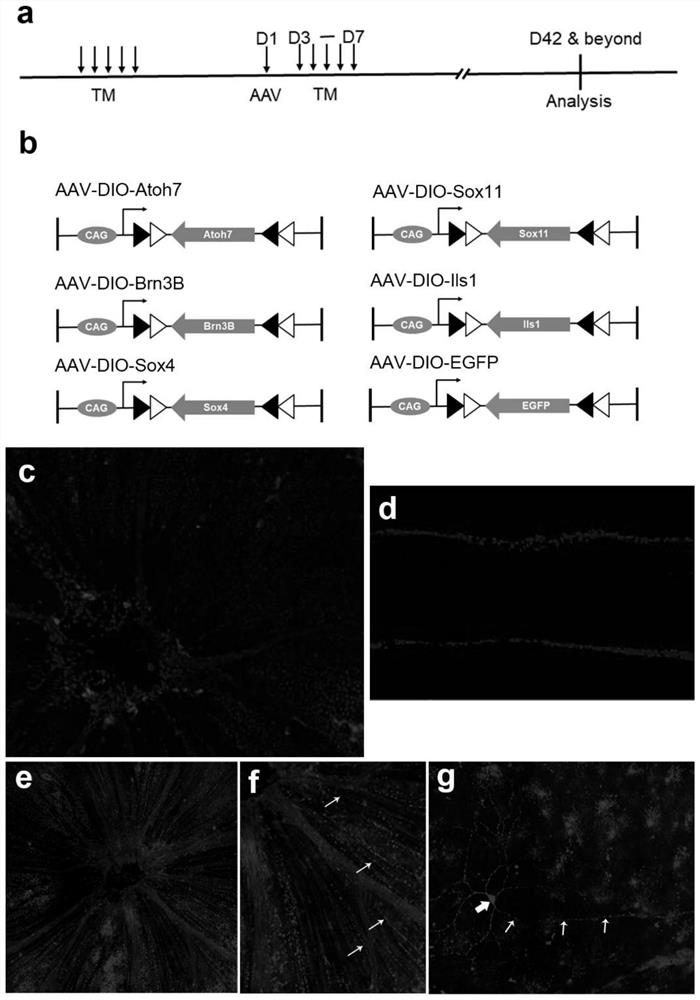
[0098] The preparation method of retinal ganglion cells in one embodiment of the present invention at least includes the following steps: applying transcription factors to adult cells to transform them into retinal ganglion cells; the transcription factors are selected from Brn3B, Sox4, Atoh7, Sox11 and Isl1 any one or more of.
[0099] In the present invention, unless otherwise specified, the pharmaceutical dosage form is not particularly limited, and can be made into dosage forms such as injections, oral liquids, tablets, capsules, dripping pills, sprays, etc., and can be prepared by conventional methods. The choice of drug dosage form should match the mode of administration.
[0100] The effective amount of the pharmaceutical preparation of the present invention should take into account factors such as the route of administration, the health status of the patient, etc., which are within the skill of skilled physicians.
[0101] Further, the adult cells are selected from on...
Embodiment 1
[0134] 1.1 Mice and feeding methods:
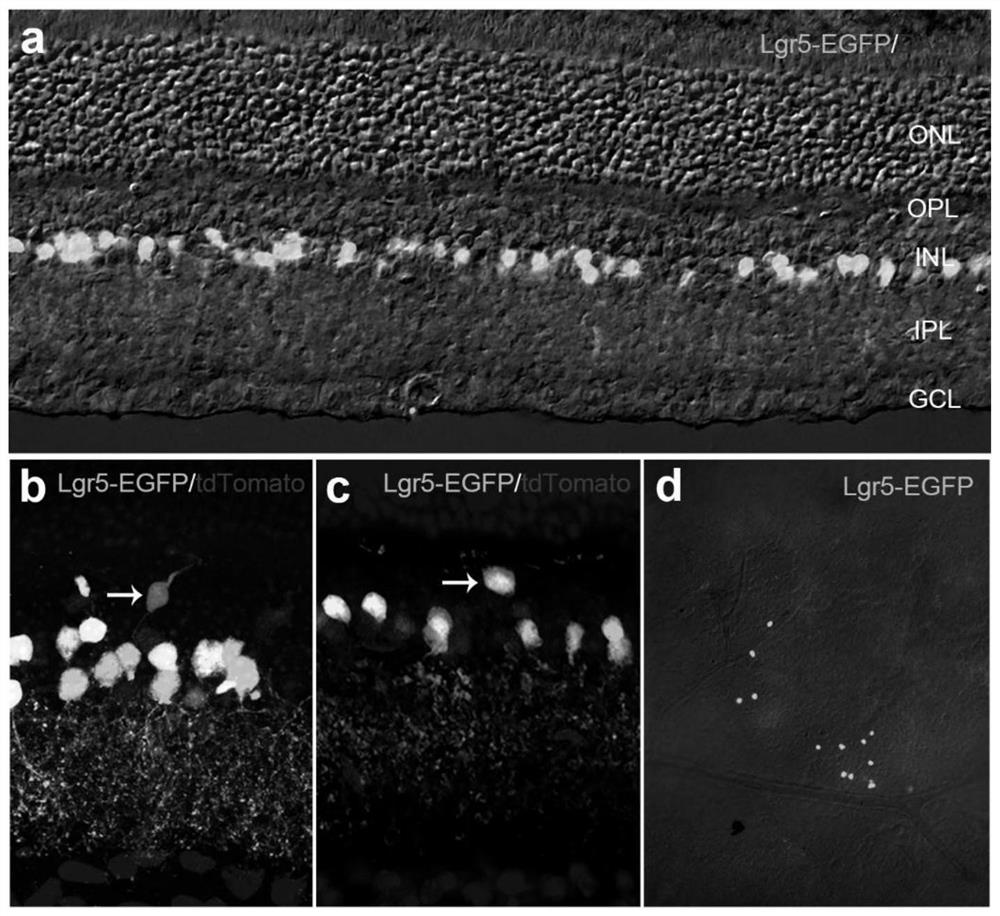
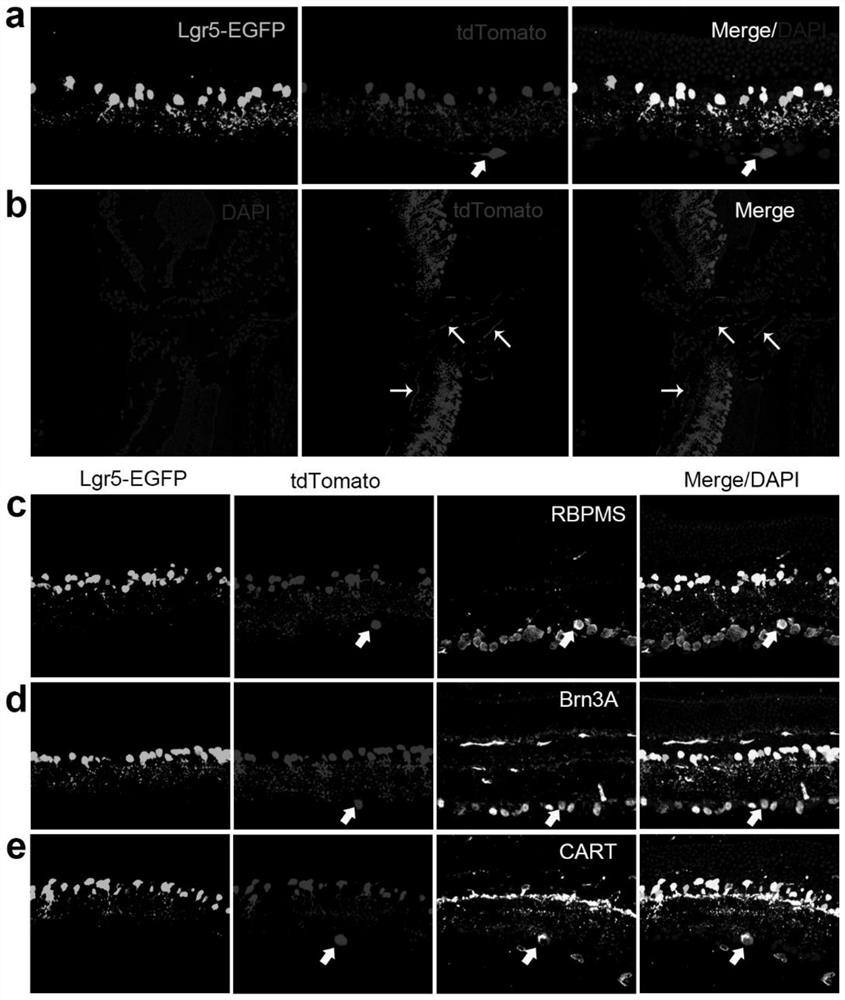
[0135] Lgr5 used in the present invention EGFP-IRES-CreERT2 , Pvalb CreERT2 , and the Rosa26-tdTomato mouse strain were purchased from Jackson laboratory. Lgr5 EGFP-IRES-CreERT2 Strain mice and Pvalb CreERT2 Strain mice were crossed with Rosa26-tdTomato mice to obtain Lgr5 EGFP-IRES-CreERT2 ; Rosa26-tdTomato mice and Pvalb CreERT2 ; Rosa26-tdTomato mice.
[0136] Prokr2 CreERT2 The mouse strain was constructed in the laboratory of ShanghaiTech University by using CRISPR / Cas9 technology through homologous recombination. The mouse was knocked into the CreERT2-PolyA expression box at the ATG site of the Prokr2 gene. The brief process of mouse construction is as follows: a vector (donor vector) containing CreERT2-PolyA expression cassette and homologous recombination arm was constructed by In-Fusion cloning method. Cas9 mRNA, gRNA and donor vector were microinjected into fertilized eggs of C57BL / 6J mice, and the injected fertilized eg...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com