Method for assigning antibody standard substance and determining antigen neutralization equivalent
一种标准品、抗体的技术,应用在医学检测领域,能够解决抗体检测试剂最低检出限无从精准确定等问题,达到提高检测准确性、提高临床效率、试剂质量稳定的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: The assignment method of antibody standard substance, specifically comprises the following steps:
[0028] Determination of antigen neutralization equivalent of S1 antibody standard: Dilute the antigen of known purity and concentration with matrix to obtain a series of gradient dilutions of different concentrations; add an equal amount of Antibody standard, after the reaction, use the first antibody detection reagent to detect the antibody of each mixed solution, so as to determine the antigen neutralization equivalent of the antibody standard, so as to assign a value to the antibody standard; among them, the amount of antibody in the antibody standard Values are expressed in mass-volume concentration units of their antigen neutralizing equivalents.
[0029] This embodiment is to prepare the antibody standard substance (standard substance) of novel coronavirus (COVID-19), and its assigning method is:
[0030] Collect high-titer antibody serum from conva...
Embodiment 2
[0034] Embodiment 2: The method for determining the minimum detection limit of antibody detection reagents specifically includes the following steps:
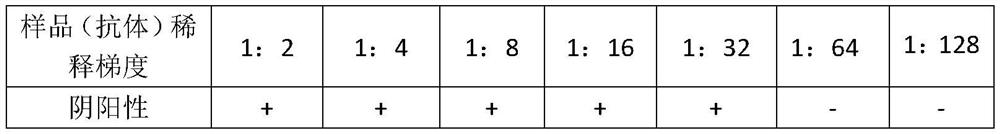
[0035] Determination of antigen neutralization equivalent of S1 sample: The antigen with known purity and concentration is serially diluted with matrix, and the dilution ratios of the serial dilution are 1:2, 1:4, 1:8, 1:16, 1:32 respectively , 1:64, 1:128, and 1:256 to obtain multiple gradient dilutions with different concentrations; add an equal amount of samples containing specific antibodies to each gradient dilution, react at 37°C for 30 minutes, and then test each Each mixed solution is detected with a specific antibody detection reagent (primary antibody detection reagent). When a positive reaction occurs in a certain dilution of the mixed solution in the test result, the antigen of the previous higher concentration mixed solution of the mixed solution is determined. The amount is the antigen neutralization equivalent of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com