Application of irisin in the preparation of medicine for the treatment of aplastic anemia
An aplastic and irisin technology, applied in the field of medicine, can solve problems such as the application of aplastic anemia without irisin, and achieve good histocompatibility
Active Publication Date: 2022-07-26
THE SECOND HOSPITAL OF SHANDONG UNIV
View PDF3 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0004] Irisin can be produced by various tissue cells such as skeletal muscle, cardiac muscle, liver, kidney, nerve, fat, pancreas, etc. Current research mainly focuses on the application of irisin in improving obesity and other metabolic diseases. Currently, there is no irisin The application of Suin in the treatment of aplastic anemia
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
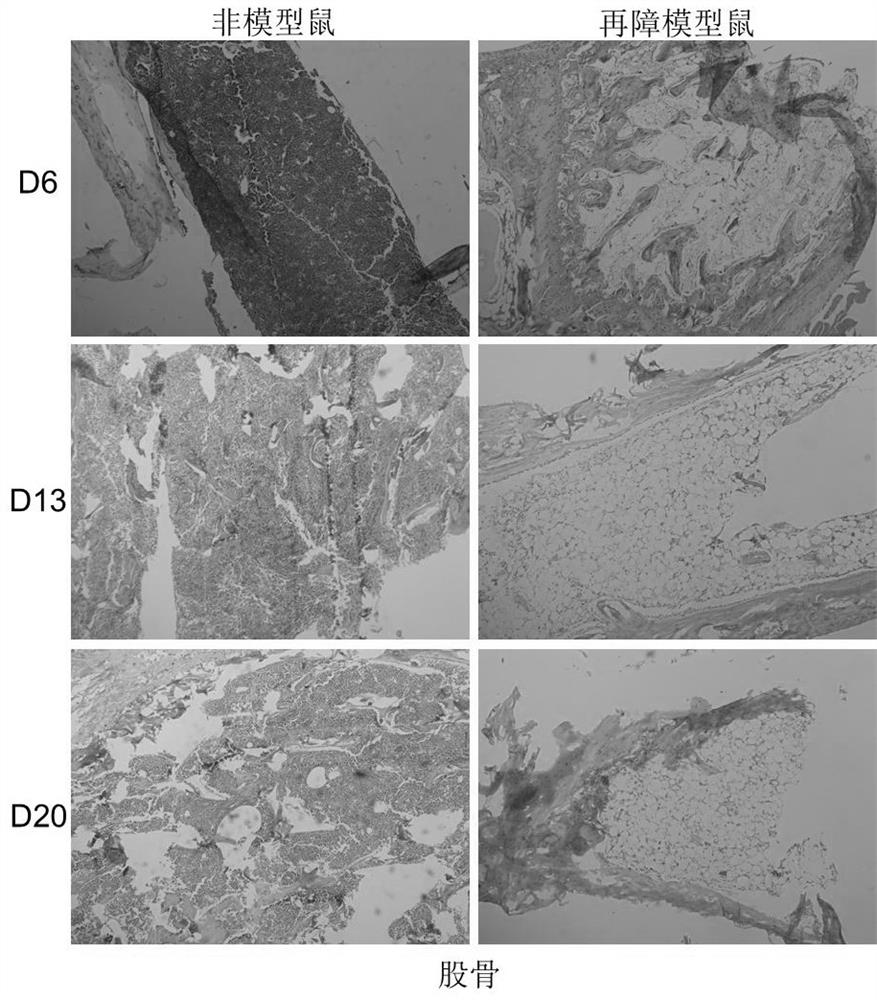
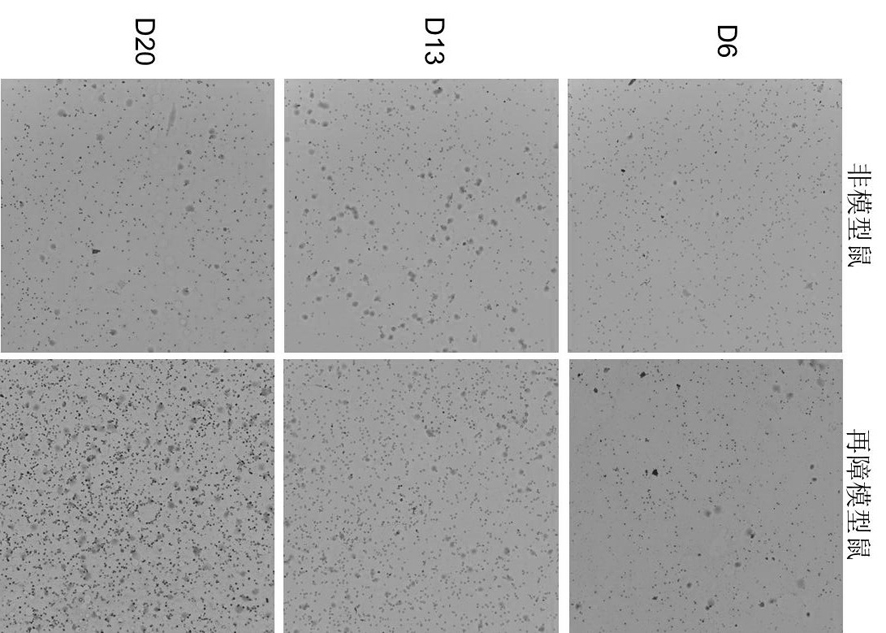
[0025] 1. Establishment of a mouse model of aplastic anemia
[0026] Using 6Gy mice total body irradiation (TBI) combined with tail vein lymphocyte infusion, the pathological features of pancytopenia and bone marrow dysplasia similar to human hematopoietic stem cell failure were replicated in mice.
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention discloses the application of irisin in the preparation of medicine for treating aplastic anemia. The administration method is intraperitoneal injection, and the dosage is 0.5 μg / g; Because irisin has no species specificity and good histocompatibility, it has no immune rejection in patients with aplastic anemia; it is not only suitable for immune cell-mediated aplastic anemia, but also suitable for non-immune-mediated aplastic anemia Acute aplastic anemia, especially hereditary anemia, provides a new intervention target for the preparation of aplastic anemia drugs. The application of irisin to the development of various types of aplastic anemia-related drugs can provide a new therapeutic target for aplastic anemia. The research on drugs for aplastic anemia provides a new choice and idea, and broadens the field of choice for aplastic anemia drugs.
Description
technical field [0001] The invention relates to the technical field of medicine, in particular to the application of irisin in the preparation of a medicine for treating aplastic anemia. Background technique [0002] Aplastic anemia is a disease caused by the severe lack of hematopoietic stem and progenitor cells and the failure of bone marrow hematopoietic function, leading to peripheral pancytopenia in patients. Clinically, anemia, hemorrhage and infection are the main manifestations. Bone marrow lesions are mainly reduced hematopoietic tissue, the total volume of red bone marrow is reduced, replaced by adipose tissue, so fat replacement of hematopoietic cells is a unique pathological feature of aplastic anemia. Almost all sporadic aplastic anemias, especially severe and acute aplastic anemias, are thought to be immune cell-mediated. Therefore, the current treatment of aplastic anemia, clinical immunosuppressive therapy, such as antithymocyte globulin (ATG), antilymphocyt...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): A61K38/22A61P7/06A61P7/00
CPCA61K38/22A61P7/06A61P7/00
Inventor 唐东起郑成云孔德晓李慧
Owner THE SECOND HOSPITAL OF SHANDONG UNIV
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Patsnap Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com