Linolenic acid-metronidazole compound and its preparation method and application
A technology of metronidazole and linolenic acid, which is applied in the field of medicine, can solve the problems of easy drug resistance, and achieve the effects of stable acidic environment, high specificity of action, and low resistance to drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
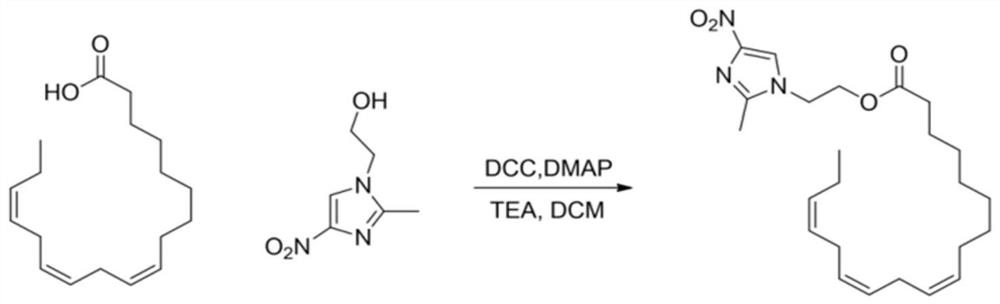
[0023] Step 1: Add 139mg of linolenic acid, 5ml of DCM, and 103mg of DCC condensing agent into a 25ml reaction bottle, stir at room temperature for 5 minutes, add 94mg of metronidazole, 0.5mL of triethylamine as a base, and 5mg of DMAP as a catalyst, and react at room temperature overnight. The molar ratio of raw materials linolenic acid and metronidazole is 1:1.1.
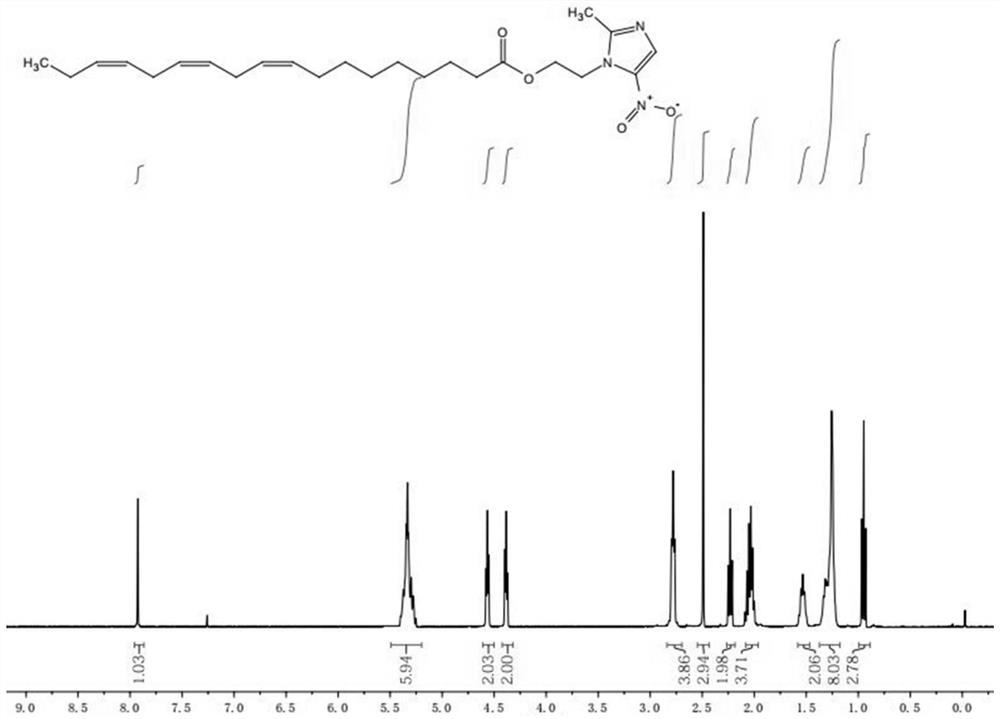
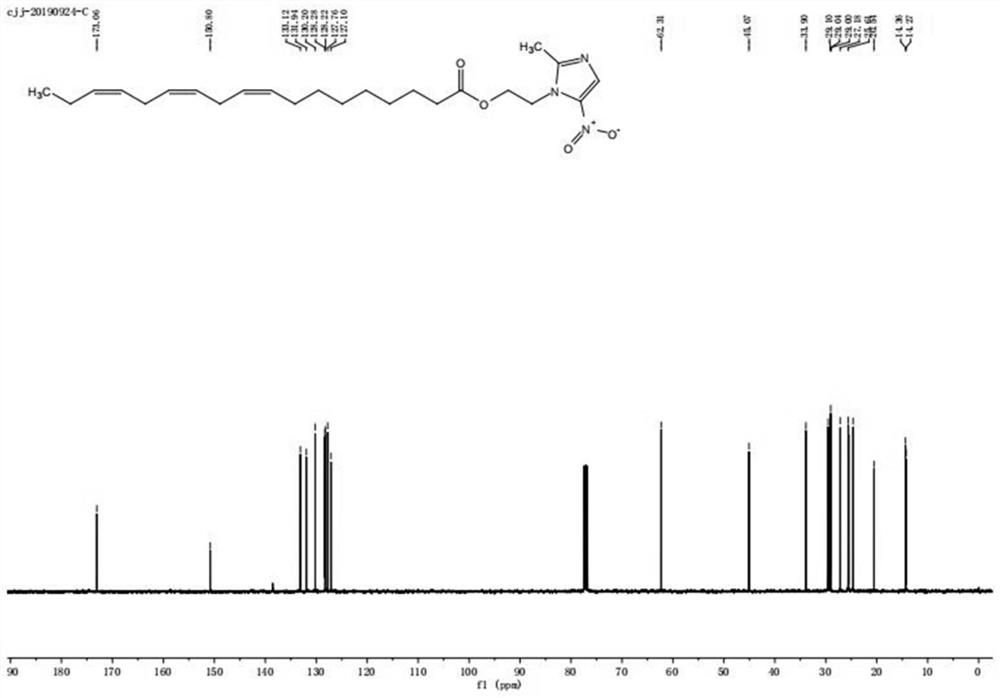
[0024] Step 2: TLC detects that the raw material of linolenic acid disappears completely, and white precipitate precipitates out of the system, and is filtered with diatomaceous earth. After the filtrate was spin-dried, column chromatography gave linolenic acid-metronidazole, about 105 mg, as a yellow oily liquid, yield: 48.7%. The product was identified as linolenic acid-metronidazole by NMR, mass spectrometry and infrared.
Embodiment 2
[0026] Step 1: Add 139mg of linolenic acid, 5ml of DCM, and 106mg of DCC condensing agent into a 25ml reaction bottle, stir at room temperature for 8 minutes, add 104mg of metronidazole, 0.6mL of triethylamine as a base, and 5mg of DMAP as a catalyst, and react at room temperature overnight. The molar ratio of raw materials linolenic acid and metronidazole is 1:1.2.
[0027] Step 2: TLC detects that the raw material of linolenic acid disappears completely, and white precipitate precipitates out of the system, and is filtered with diatomaceous earth. After the filtrate was spin-dried, column chromatography gave linolenic acid-metronidazole, about 102.8 mg, as a yellow oily liquid, yield: 45.3%. The product was identified as linolenic acid-metronidazole by NMR, mass spectrometry and infrared.
Embodiment 3
[0029] Step 1: Add 139mg of linolenic acid, 5ml of DCM, and 108mg of DCC condensing agent into a 25ml reaction bottle, stir at room temperature for 6 minutes, add 94mg of metronidazole, 0.6mL of triethylamine as a base, and 5mg of DMAP as a catalyst, and react at room temperature overnight. The molar ratio of raw materials linolenic acid and metronidazole is 1:1.1.
[0030] Step 2: TLC detects that the raw material of linolenic acid disappears completely, and white precipitate precipitates out of the system, and is filtered with diatomaceous earth. After the filtrate was spin-dried, column chromatography gave linolenic acid-metronidazole, about 103 mg, as a yellow oily liquid, yield: 46.5%. The product was identified as linolenic acid-metronidazole by NMR, mass spectrometry and infrared.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com