Use and modification method of modified peptide in preparation of drug for inhibiting insulin secretion from pancreatic islets
An insulin and pancreatic islet technology, applied in the field of peptide modification, can solve the problems of patient suffering, unstable polypeptide drugs, affecting the application of COX, etc., and achieve the effect of enhancing resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
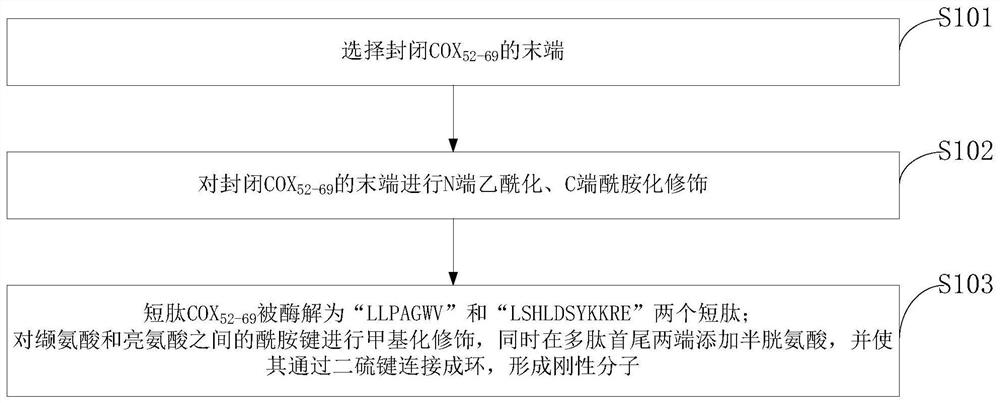
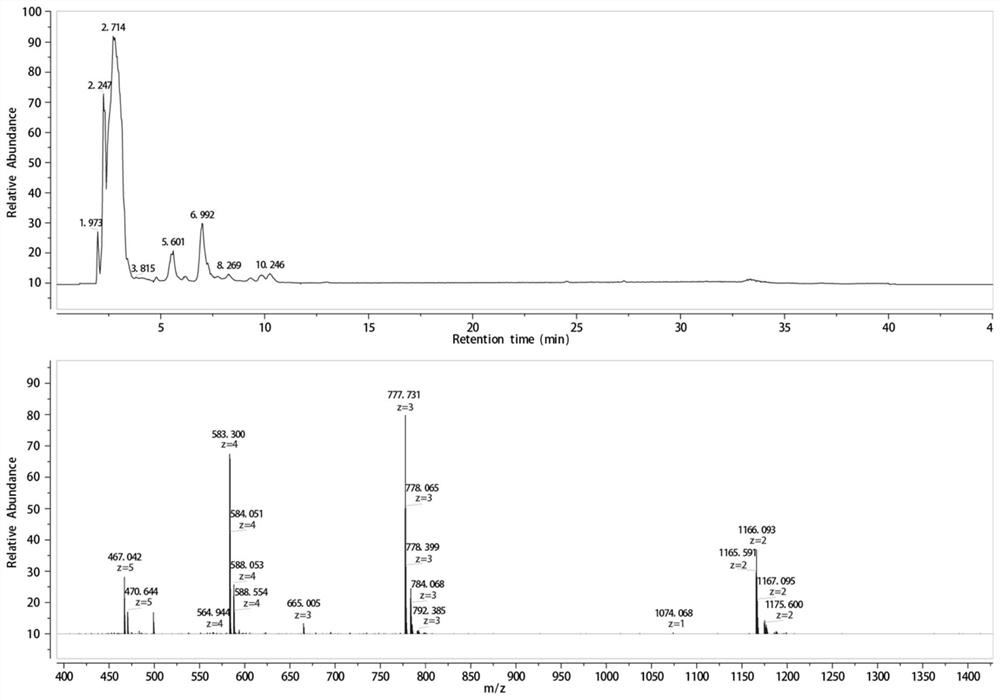
[0052] First select closed COX 52-69 N-terminal acetylation, C-terminal amidation modification, reducing the total charge at both ends of the polypeptide, making it closer to the parent protein, and enhancing resistance to endopeptidases. After incubation with simulated gastrointestinal fluid in vitro, it was found by HPLC-MS that the short peptide COX 52-69 It is digested into two short peptides "LLPAGWV" and "LSHLDSYKKRE".
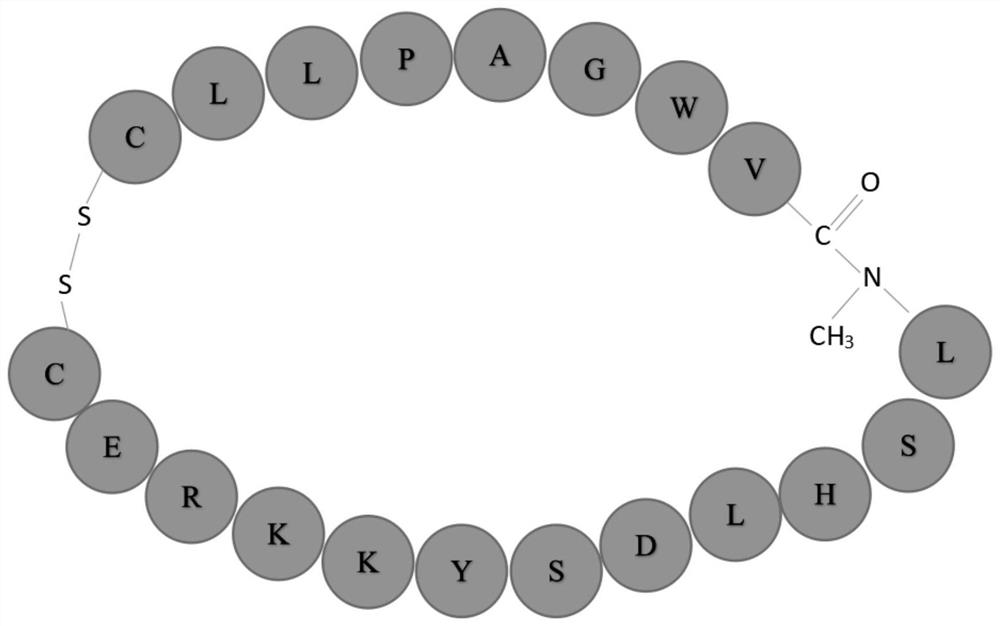
[0053] In order to make it stably exist in the simulated gastrointestinal fluid, the present invention methylates the amide bond between valine and leucine, and adds cysteine at both ends of the polypeptide at the same time, and makes it pass through two The sulfur bonds connect to form a ring, making it a rigid molecule. After incubation with simulated gastrointestinal fluid in vitro, it was found by HPLC-MS that the short peptide COX 52-69 Can exist stably in simulated gastrointestinal fluid.
Embodiment 2
[0055] To confirm the modified short peptide COX 52-69 It still has the function of inhibiting glucose-induced insulin secretion, and the COX modified by methylation and cyclization was detected by ELISA. 52-69 Tissue supernatants from the incubated islets were assayed. It can be seen from the statistical results that the modified peptide COX 52-69 It still has the function of inhibiting glucose-induced insulin secretion.
[0056] At the same time, in order to verify the modified peptide COX 52-69 Through the intestinal mucosa, the present invention constructs the Caco-2 cell model in vitro, and the modified peptide COX 52-69 The transmembrane transport ability was tested. The modified peptide COX was found by evaluating its apparent permeability coefficient (Papp) 52-69 Good transmembrane transport ability.
[0057] It can be confirmed by the above experiments that the modified peptide COX 52-69 It still has the function of inhibiting glucose-induced insulin secretion,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com