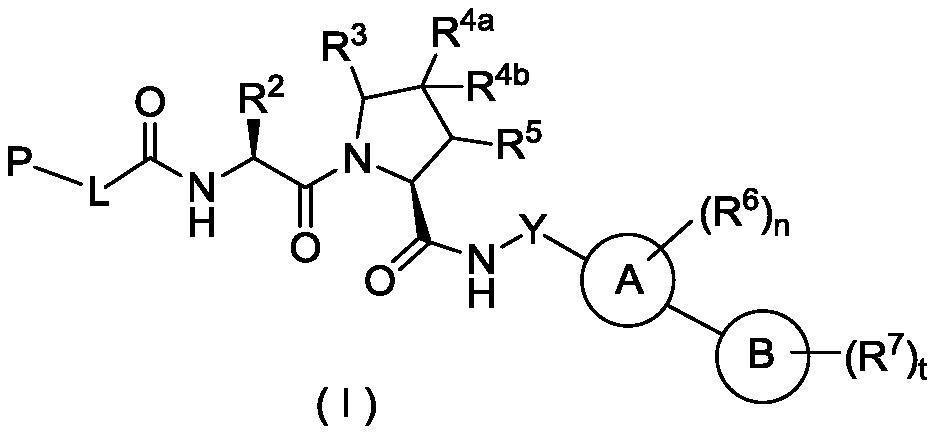
Hydroxyproline derivative for preparing proteolysis targeting chimeras (PROTACs)
A technology of compounds and mesoforms, applied in the field of protein degradation targeting chimera compounds, can solve the problems of poor water solubility of fulvestrant, increased dosage, and low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0490] (2S,4R)-1-((S)-2-Acetamido-3,3-dimethylbutyryl)-4-hydroxy-N-(1-(4-(4-methylthiazole-5- Base) phenyl) cyclobutyl) pyrrolidine-2-carboxamide 1
[0491]
[0492]
[0493] first step
[0494] (1-(4-(4-methylthiazol-5-yl)phenyl)cyclobutyl)carbamate tert-butyl ester 1b
[0495] (1-(4-Bromophenyl) cyclobutyl) tert-butyl carbamate (300mg, 0.92mmol, Shanghai Bi De Pharmaceutical Technology Co., Ltd.) and palladium acetate (20.6mg, 0.092mmol), potassium acetate (181mg, 1.84mmol), 4-methylthiazole (182mg, 1.84mmol) was dissolved in N,N-dimethylacetamide (6mL), and heated to 90°C under the protection of argon to react overnight. Concentrate under reduced pressure to remove the solvent, and purify by thin-layer chromatography with developer system B to obtain the title compound 1b (300 mg), yield: 94%.
[0496] MS m / z(ESI):345[M+1]
[0497] second step
[0498] 1-(4-(4-methylthiazol-5-yl)phenyl)cyclobutan-1-amine hydrochloride 1c
[0499] Compound 1b (300mg, 0.87mmol) wa...
Embodiment 2
[0521] (2S,4R)-1-((S)-2-Acetamido-3,3-dimethylbutyryl)-4-hydroxy-N-(3-(4-(4-methylthiazole-5- Base) phenyl) oxetan-3-yl) pyrrolidine-2-carboxamide 2
[0522]
[0523]
[0524] first step
[0525] 2-Methyl-N-(3-(4-(4-methylthiazol-5-yl)phenyl)oxetan-3-yl)propane-2-sulfinamide 2b
[0526] N-(3-(4-bromophenyl)oxetan-3-yl)-2-methylpropane-2-sulfinamide 2a (300mg, 0.903mmol, using a known method Org.Lett., 2011 , 13,3912-3915) and 4-methylthiazole (179mg, 1.81mmol), palladium acetate (20mg, 0.090mmol), pivalic acid (28mg, 0.027mmol), potassium carbonate (187mg, 1.35mmol) , Tricyclohexylphosphine tetrafluoroborate (33mg, 0.090mmol) was mixed and dissolved in N,N-dimethylformamide (10mL), filled with argon, and heated to 110°C for 17 hours. After the reaction solution was cooled to room temperature, it was diluted with water, extracted with ethyl acetate, washed with saturated sodium chloride solution, and dried over anhydrous sodium sulfate. Filtrate, concentrate under red...
Embodiment 3
[0550] (2S,4R)-1-((S)-2-Acetamido-3,3-dimethylbutyryl)-4-hydroxy-N-(1-(4-(4-methylthiazole-5- Base) phenyl) cyclopropyl) pyrrolidine-2-carboxamide 3
[0551]
[0552] first step
[0553] (1-(4-(4-methylthiazol-5-yl)phenyl)cyclopropyl)carbamate tert-butyl ester 3b
[0554] (1-(4-bromophenyl)-cyclopropyl) tert-butyl carbamate 3a (250mg, 0.801mmol, Shanghai Bi De Pharmaceutical Co., Ltd.), 4-methylthiazole (159mg, 1.60mmol), acetic acid Palladium (18mg, 0.08mmol) and potassium acetate (157mg, 1.60mmol) were mixed and dissolved in N,N-dimethylacetamide solvent (10mL). The reaction was heated to 100°C and stirred overnight. The reaction was diluted with water (20 mL), extracted with ethyl acetate (20 mL×3), the organic phases were combined, washed with saturated sodium chloride solution (20 mL), and dried over anhydrous sodium sulfate. Filtrate, concentrate under reduced pressure to remove the solvent, and purify by thin-layer chromatography with developer system B to obtain ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com