Drug delivery devices and systems for local drug delivery to the upper urinary tract
A technology for delivering devices and drugs, which can be used in drug delivery, drug devices, drug formulations, etc., and can solve problems such as invasiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0186] Example 1 - Drug Delivery Device
[0187] Preparation of gemcitabine release devices for in vitro testing. Use bi-material polyurethane tubing to construct the system. The inner cavity of the drug reservoir of the drug delivery device of this embodiment contains a gemcitabine hydrochloride powder blend, which includes gemcitabine hydrochloride, 30 polyvinylpyrrolidone (PVP) (BASF Corp., USA) and Fumed silica (Cabot Corp., USA).
[0188] The drug delivery device has a dual lumen structure comprising a substantially circular guidewire lumen and a drug reservoir lumen having a crescent cross-sectional shape such as Figure 1C Lumen of drug reservoir shown. The elastic body of the device is made of barium sulfate loaded tecoflex polyurethane (TECOFLEX TM EG-80A-B20, 20% tecoflex polyurethane loaded with barium sulfate (Lubrizol Life Sciences, USA)) and TECOPHILIC TM Made of TPU polyurethane (HP-60D-35 (Lubrizol Life Sciences, USA)). tecoflex polyurethane is a w...
Embodiment 2
[0194] Example 2 - In Vivo Testing of Drug Delivery Devices
[0195] An in vivo test was used to evaluate the migration of two drug delivery devices with different retention shapes.
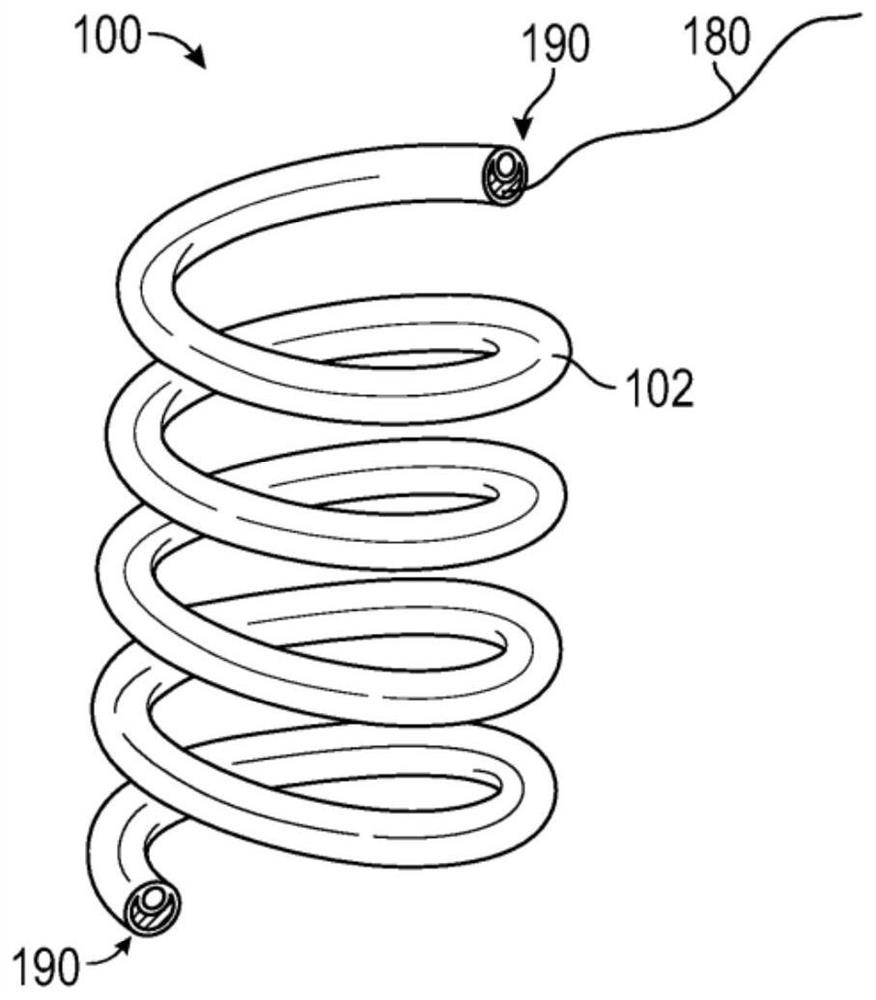
[0196] A single coil design (e.g., figure 2 of those) deployed into the poles of the renal pelvis of the kidneys of domestic Yorkshire pigs. Upon necropsy, the drug delivery devices were found to migrate, and smaller drug delivery devices were found to be more prone to migration than larger drug delivery devices. Additionally, the length of the single coil drug delivery device is relatively short in order to fit in the available renal pelvis space for the limited drug payload.
[0197] Single coil drug delivery devices were placed in the upper and lower poles of four kidneys of four domestic Yorkshire pigs. Two small (i.e., 4 cm) single coil drug delivery devices were deployed in two animals for 10 days (total of four small drug delivery devices), and two large (i.e., 6 cm) were deployed in...
Embodiment 3
[0202] Example 3 - Drug Delivery Device Shape Changes
[0203] prepared as Image 6 A drug delivery device is shown wherein the helical portion has three turns and the straight end extends generally perpendicular to the helical portion of the device. The straight ends are shaped by heat setting and aligned diagonally across the device. The outer diameter of the coil is 12mm.
[0204] prepared as Figure 7 Drug delivery device shown where the helical portion has six turns and the straight end is formed by heat setting. The outer diameter of the coil of the device is 8 mm. The uncoiled length of the device was 120 mm.
[0205] prepared as Figure 8 Drug delivery device shown with three coils spaced apart by two straight mid-sections. The uncoiled length of the device was 85mm.
[0206] prepared as Figure 9 Drug delivery device shown with two coils separated by a single straight middle section. The uncoiled length of the device was 75mm.
[0207] A drug delivery dev...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| Shore hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com