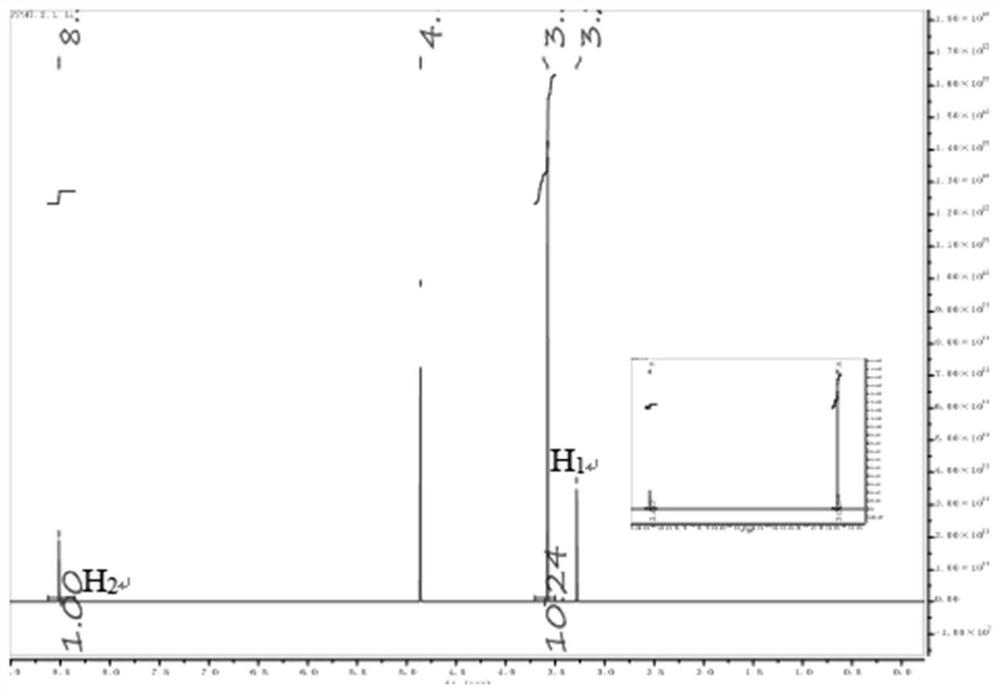
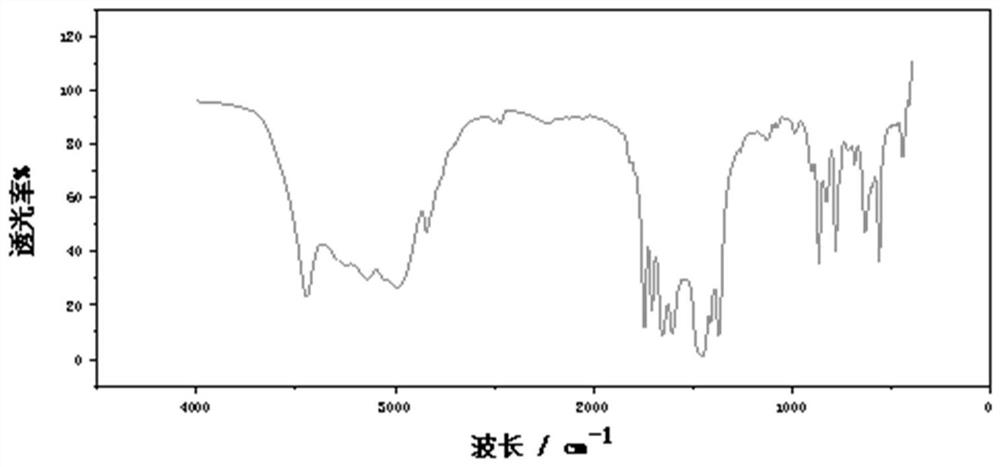
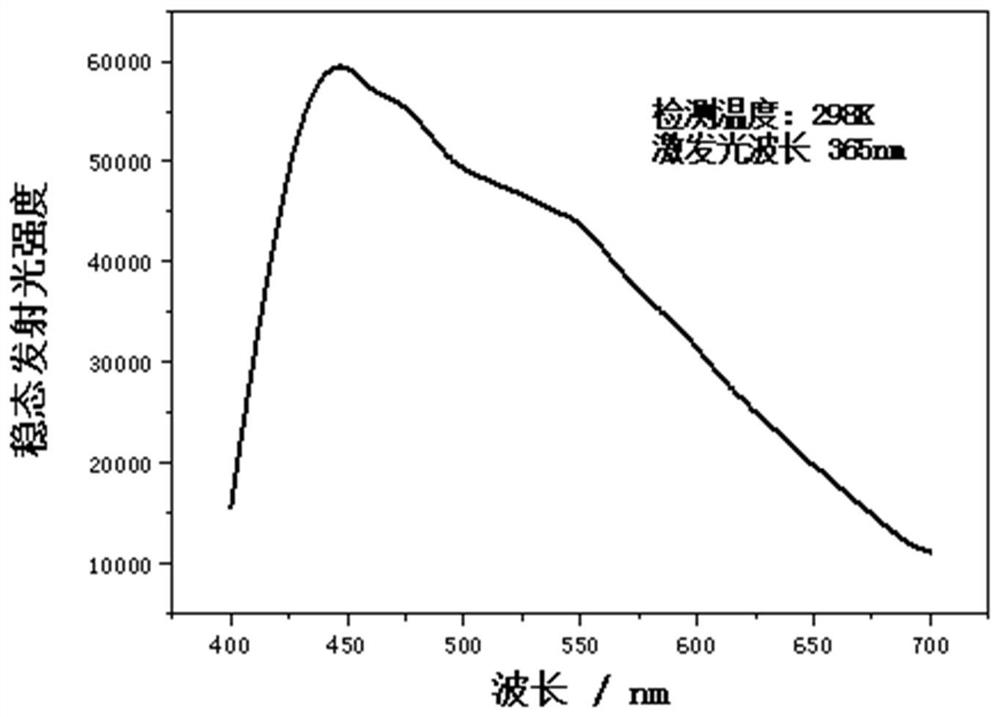
Triazine polymer and preparation method thereof
A technology of polymers and triazines, applied in the field of triazine polymers, can solve the problems of low solubility and insufficient afterglow characteristics, and achieve the effects of long phosphorescence life, good long afterglow characteristics, and unique luminous characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] A kind of triazine polymer and preparation thereof:
[0049] 1) Compounds containing s-triazine: At 50°C, tripolychlorazine was added to sodium bicarbonate sodium bicarbonate-sodium carbonate buffer solution (pH 8.0) for hydrolysis to obtain 2-hydroxy-4,6- Dichloro-s-triazine;
[0050] Described sodium bicarbonate-sodium carbonate buffer solution is that sodium bicarbonate and sodium carbonate buffer solution are mixed to obtain at 2:1;
[0051] 2) Diol disodium salt: Add ethylene glycol into the reaction solvent 1,4-dioxane, mix and stir, put it in an ice bath after the dissolution is complete, and add NaH in batches after the reaction solution drops to 0°C , the ratio of ethylene glycol and NaH is 1:2.5, react until no bubbles are generated;
[0052] 3) Triazine polymer: In a dry and airtight environment at 25°C, mix 2-hydroxy-4,6-dichloro-s-triazine and diol disodium salt at a ratio of 1:1.2 and react at room temperature for 48 hours to obtain white The turbid liq...
Embodiment 2
[0063] A kind of triazine polymer and preparation thereof:
[0064] 1) Compounds containing s-triazine: Add tripolychlorazine to sodium bicarbonate-sodium carbonate buffer solution (pH 8.5) for hydrolysis at 50°C to obtain 2-hydroxy-4,6-dichloro-s-triazine triazine;
[0065] Described sodium bicarbonate-sodium carbonate buffer solution is that sodium bicarbonate and sodium carbonate buffer solution are mixed to obtain at 2:1;
[0066] 2) Diol disodium salt: Add propylene glycol into the reaction solvent anhydrous tetrahydrofuran, mix and stir, put it in an ice bath after the dissolution is complete, and add NaH in batches after the reaction solution drops to 0°C, and the propylene glycol and NaH The ratio is 1:3, react until no bubbles are generated;
[0067] 3) Triazine polymer: In a dry and airtight environment at 35°C, mix 2-hydroxy-4,6-dichloro-s-triazine and diol disodium salt at a ratio of 1:1.3 and react at room temperature for 48 hours to obtain white The turbid liq...
Embodiment 3
[0069] A kind of triazine polymer and preparation thereof:
[0070] 1) Diol disodium salt: add polyether diol to the reaction solvent 1,4-dioxane, mix and stir, put it in an ice bath after it is completely dissolved, and add it in batches after the reaction solution drops to 0°C NaH, the ratio of described polyether glycol and NaH is 1:3.5, reacts to produce without bubble;
[0071] 2) Triazine polymer: In a dry and airtight environment at 25°C, tripolychlorazine and diol disodium salt were mixed and reacted at a ratio of 1:0.08, reacted at room temperature for 48 hours to obtain a white turbid liquid, and the mixture was centrifuged to obtain The crude product of α,ω-dihydroxypoly-2-chloro-4,6-ethyl ether-s-triazine was washed with tetrahydrofuran and deionized water respectively to remove the raw materials and oligomers to obtain pure flaky white α , ω-dihydroxypoly-2-chloro-4,6-ethyl ether-s-triazine products.
[0072] The α, ω-dihydroxyl poly 2-chloro-4,6-ethyl ether s-t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com