Radionuclide labeled FAPI compound and synthesis process method thereof
A radionuclide and synthesis process technology, applied in the field of radionuclide-labeled FAPI compounds and their synthesis processes, can solve the problems of limited clinical promotion, inability to achieve mass production and distribution, and low primary output, and achieves the ability to meet clinical needs. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] This embodiment relates to a compound Al of radionuclide-labeled FAPI 18 F-FAPI synthesis process method, wherein Al 18 The chemical structural formula of F-FAPI is:
[0031]
[0032] This example is intended to synthesize liquid nuclides 18 F-marked FAPI ( 18 F-FAPI), specifically, using Al 3 + ion pair 18 Strong complexation and bridging of F ions and cyclic coordination groups (DOTA and NOTA) to synthesize Al with longer half-life 18 F-FAPI to achieve Al 18 F-FAPI has a synthesis process of more than 99% radiochemical purity and a single yield greater than 100mCi, which meets clinical needs.
[0033] Al given in this example 18 The specific process synthesis steps of F-FAPI include:
[0034] (1) Raw material preparation stage: prepare the following doses of raw materials, including:
[0035] Reagent name dose Methyl sulfoxide (DMSO) 300μL 0.5mol / L NaOAc 0.3mL 10mmol / L AlCl 3
6μL 4mmol / L FAPI-04 20 μL Absol...
Embodiment 2
[0050] Embodiment 1 obtains the quality control method of product:
[0051] (1) Physical and chemical properties:
[0052] Color: colorless
[0053] Properties: transparent clear liquid
[0054] pH value: 4.0~6.0
[0055] (2) Radiochemical purity test:
[0056] TLC quality control:
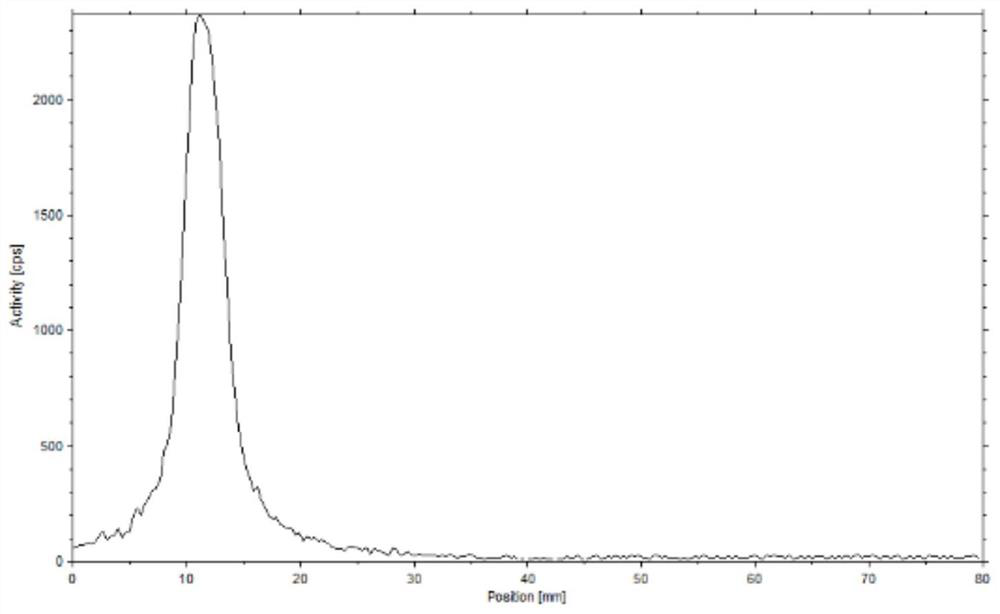
[0057] Using 0.1mol / L sodium acetate solution as developing agent, TLC was carried out to obtain quality control results:
[0058] Depend on figure 1 As shown, the product only exists at the origin position (obvious radioactive peak), but no radioactive peak appears at the front end (70mm-80mm), indicating that Al 18 The radiochemical purity of F-FAPI is close to 100%.
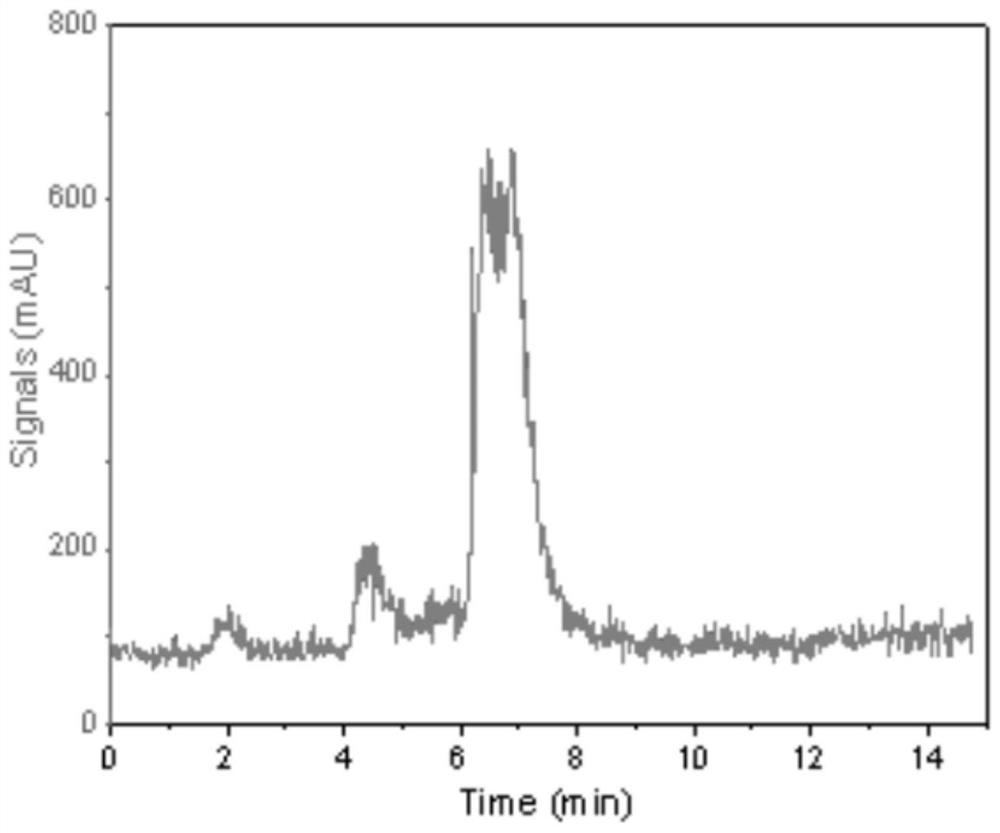
[0059] HPLC quality control:
[0060] The composition of the product was determined by high performance liquid chromatography (HPLC), and the detection parameters: ① column length: 200mm; ② column diameter: 4.6mm; ③ column packing C18; ④ mobile phase: A phase: water, B phase: acetonitrile, gradient elution: 0~10min from 95%...
Embodiment 3
[0062] Embodiment 3: the product that embodiment 1 obtains is used for biological experiment effect
[0063] (1) Experiment 1:
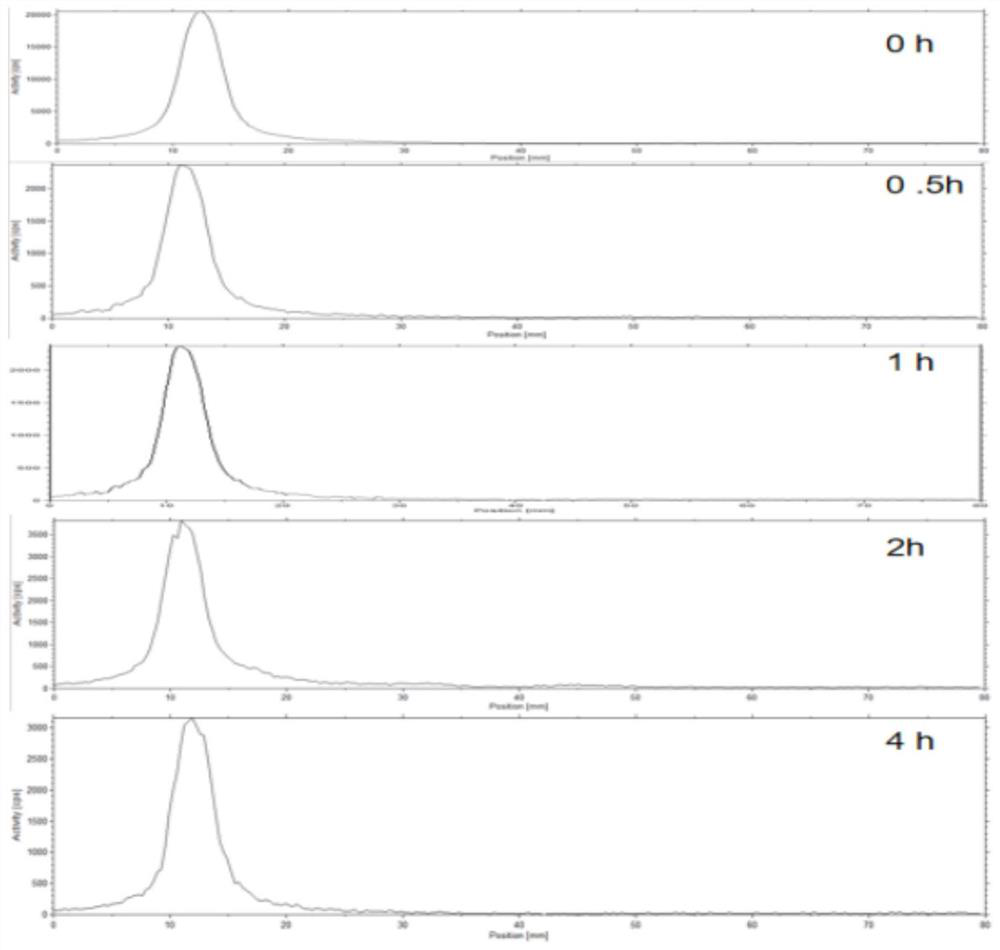
[0064] Product stability test in vitro:
[0065] A certain amount of the product was added to PBS and fresh mouse serum respectively, and the radiochemical purity was measured at different time points of 0, 0.5h, 1h, 2h, and 4h to detect the stability of the product. see results image 3 , 4h later, the radiochemical purity of the products was greater than 95%, indicating that they maintained high stability.
[0066] (2) Experiment 2:
[0067] In vivo distribution experiment in tumor-bearing nude mice:
[0068] Twelve female BALB / c-nu / nu mice, 6 weeks old, weighing about 20g, subcutaneously inoculated about 1×107 cells of SK-LMS-1 in the right leg, and injected them through the tail vein of the mice after the tumor volume reached about 1cm3 Product, 20uCi / mouse, select four time points (0.5h, 1h, 1.5h, 2h) after injection, execute 3 tumor-bearin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com