NOBIN biaryl compound and synthesis method thereof
A synthesis method and compound technology, which are applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of few reports on the construction methods of NOBIN biaryl compounds, and achieve excellent regioselectivity and good functional groups. The effect of compatibility, good yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
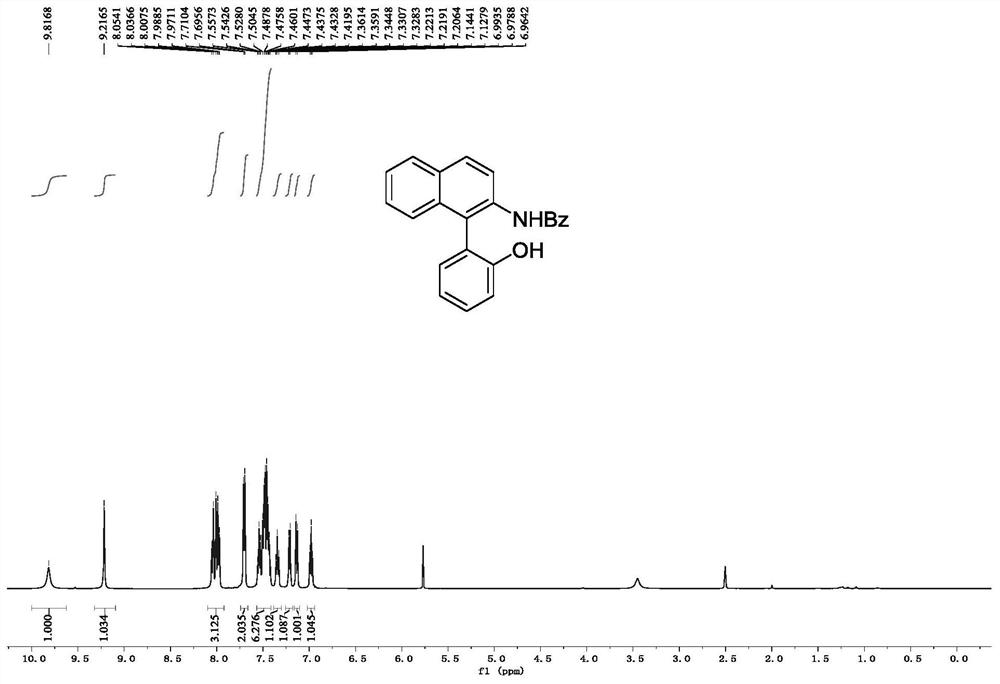
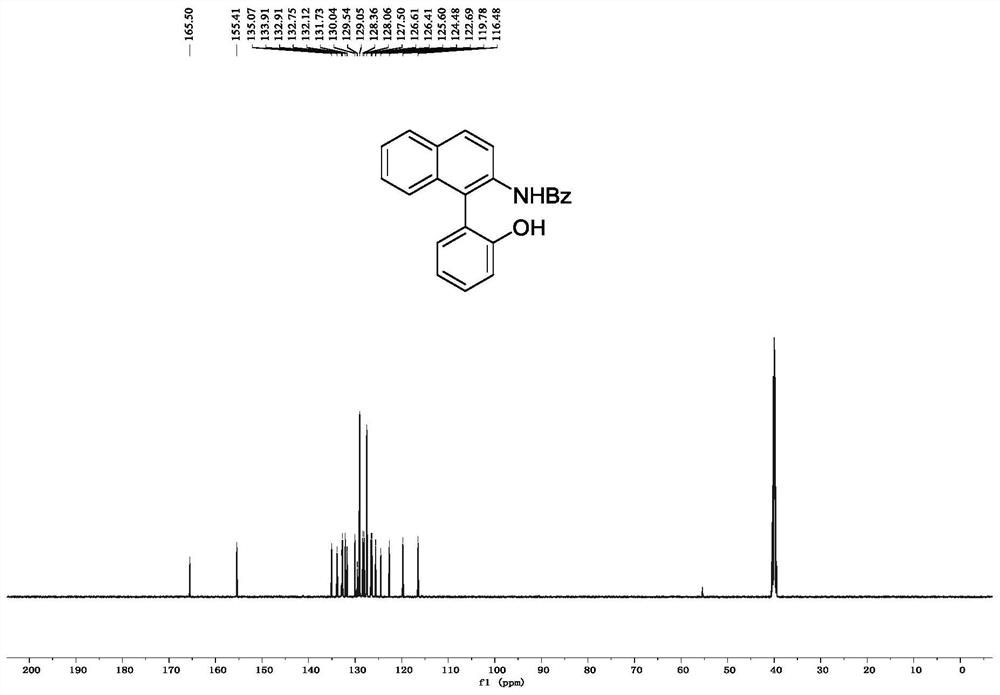
[0072] Embodiment 1, the synthesis of N-(1-(2-hydroxyphenyl) naphthalene-2-yl) benzamide
[0073]
[0074] In a 25mL reaction tube, add N-hydroxy-N-(naphthalene-2-yl)benzamide (0.2mmol, 53mg), phenylboronic acid (0.3mmol, 37mg), Cu(OTf) 2 (0.3mmol, 72mg) and Pyridine (0.3 mmol, 24 μL) was added to a solution of molecular sieves (100 mg) in DCM (2 mL), the mixture was stirred at room temperature, and the reaction progress was tracked by TLC. After the reaction was completed, the reaction mixture was filtered through diatomaceous earth, and the filtrate was washed with 3M Washed with HCl, extracted with dichloromethane, the organic phase was dried and concentrated, and the crude product was subjected to column chromatography (eluent was dichloromethane:ethyl acetate=80:1) to obtain white powdery pure product N-(1-(2 -Hydroxyphenyl)naphthalen-2-yl)benzamide, the yield was 88%.
[0075] 1 H NMR (500MHz, DMSO-d 6 ): δ9.82(s,1H),9.22(s,1H),8.10-7.93(m,3H),7.71(t,J=10.7Hz,2H)...
Embodiment 2
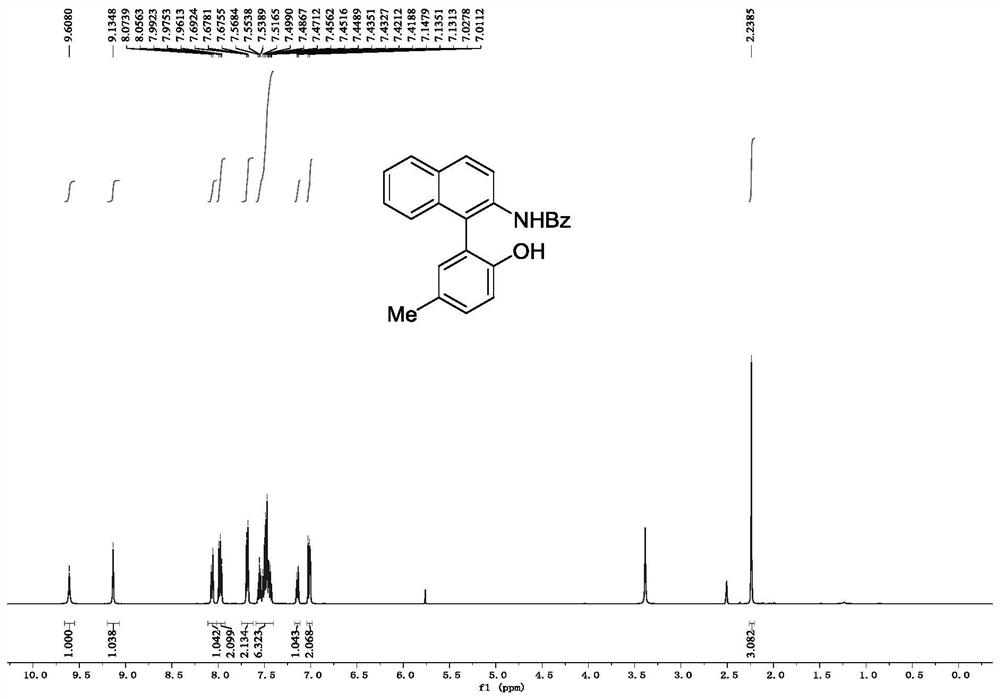
[0077] Embodiment 2, the synthesis of N-(1-(2-hydroxyl-5-methylbenzene) naphthalene-2-yl) benzamide
[0078]
[0079] In a 25mL reaction tube, add N-hydroxy-N-(naphthalene-2-yl)benzamide (0.2mmol, 53mg), p-tolylboronic acid (0.3mmol, 41mg), Cu(OTf) 2 (0.3mmol, 72mg) and Pyridine (0.3 mmol, 24 μL) was added to a solution of molecular sieves (100 mg) in DCM (2 mL), the mixture was stirred at room temperature, and the reaction progress was tracked by TLC. After the reaction was completed, the reaction mixture was filtered through diatomaceous earth, and the filtrate was washed with 3M Washed with HCl, extracted with dichloromethane, the organic phase was dried and concentrated, and the crude product was subjected to column chromatography (eluent was dichloromethane:ethyl acetate=80:1) to obtain white powdery pure product N-(1-(2 -Hydroxy-5-methylphenyl)naphthalen-2-yl)benzamide, yield 89%.
[0080] 1 H NMR (500MHz, DMSO-d 6 ): δ9.61(s,1H),9.13(s,1H),8.07(d,J=8.8Hz,1H),8.0...
Embodiment 3
[0082] Embodiment 3, the synthesis of N-(1-(2-hydroxyl-5-methoxybenzene) naphthalene-2-yl) benzamide
[0083]
[0084] In a 25mL reaction tube, add N-hydroxy-N-(naphthalene-2-yl)benzamide (0.2mmol, 53mg), p-methoxyphenylboronic acid (0.3mmol, 46mg), Cu(OTf) 2 (0.3mmol, 72mg) and Pyridine (0.3 mmol, 24 μL) was added to a solution of molecular sieves (100 mg) in DCM (2 mL), the mixture was stirred at room temperature, and the reaction progress was tracked by TLC. After the reaction was completed, the reaction mixture was filtered through diatomaceous earth, and the filtrate was washed with 3M Washed with HCl, extracted with dichloromethane, the organic phase was dried and concentrated, and the crude product was subjected to column chromatography (eluent was dichloromethane:ethyl acetate=80:1) to obtain white powdery pure product N-(1-(2 -Hydroxy-5-methoxyphenyl)naphthalen-2-yl)benzamide, yield 66%.
[0085] 1 H NMR (500MHz, DMSO-d 6 ):δ9.33(s,1H),9.25(s,1H),8.05-7.92(m,3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com