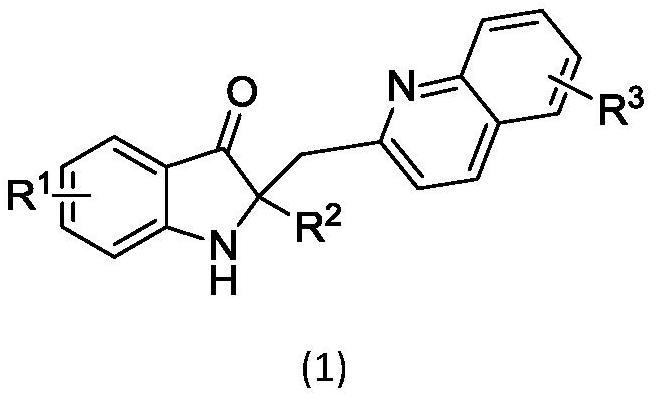
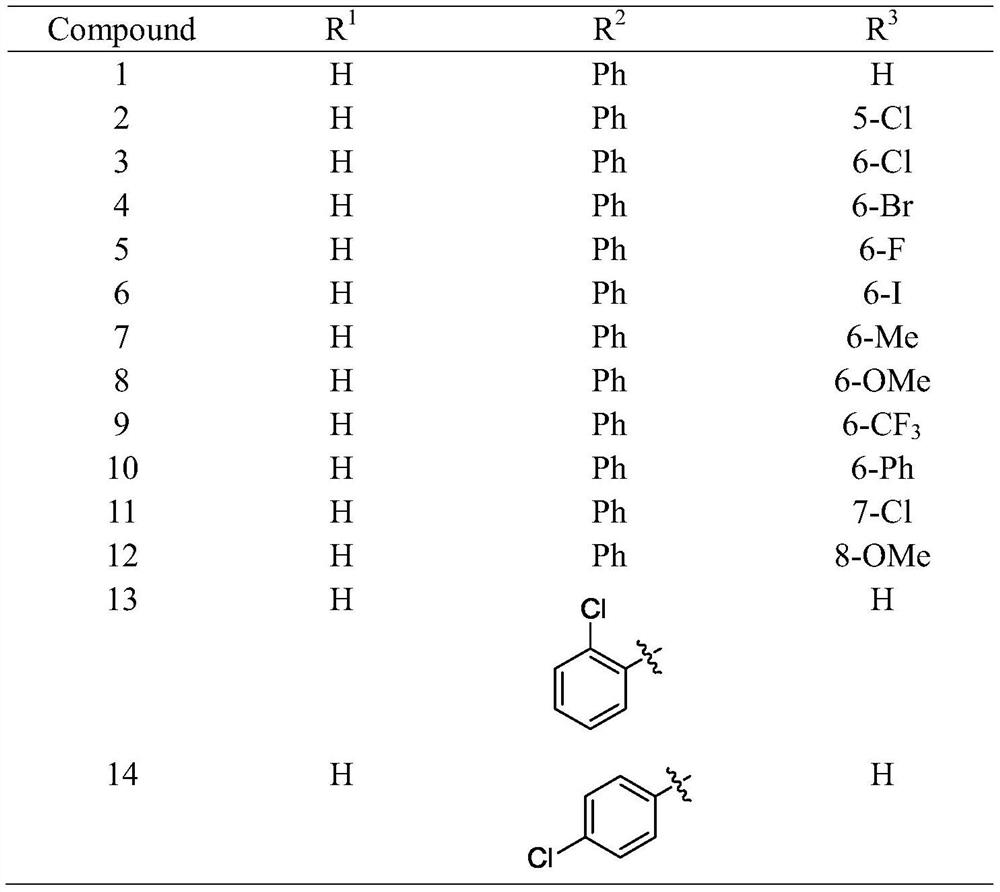
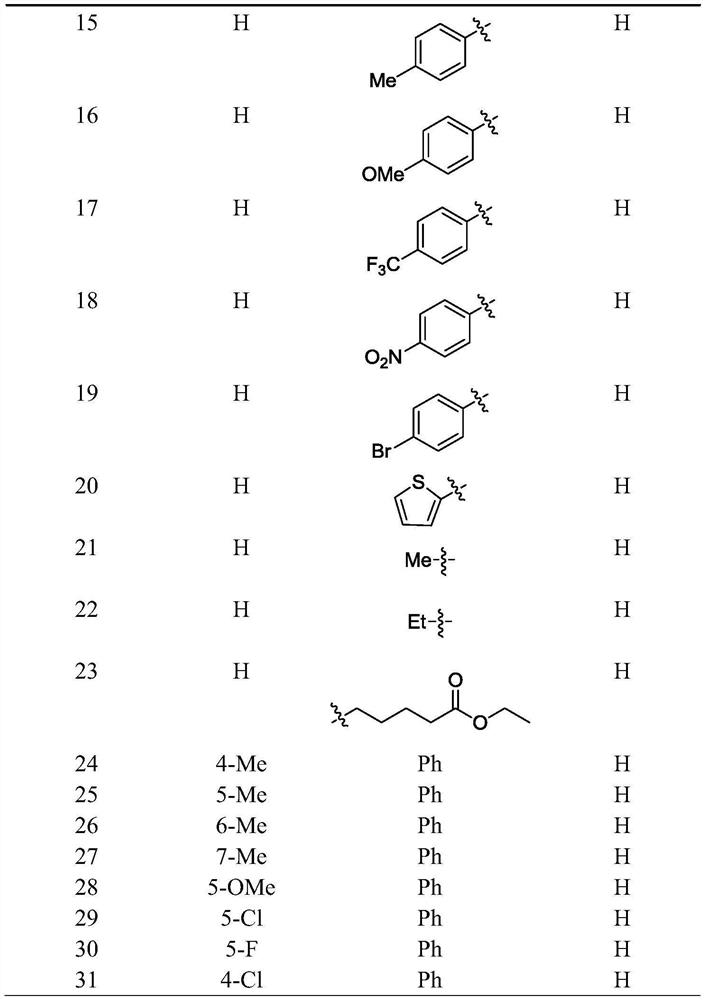
2, 2-disubstituted indoline-3-ketone alkaloid as well as preparation method and application thereof
A technology of indoline and alkaloids, applied in organic chemistry, antibacterial drugs, etc., can solve the problems of industrial application limitations, toxicity, etc., and achieve the effects of simple operation, wide substrate range, and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Preparation of 2-phenyl-2-(2-quinolylmethyl)indolin-3-one:
[0055] 2-Phenylindole (38.6mg, 0.2mmol) was placed in a 10ml dry round-bottomed flask containing a magneton, and anhydrous ethyl acetate (4ml), cuprous chloride (2.0mg, 0.02mmol), 2-Methylquinoline III (57.3mg, 0.4mmol), pivalic acid (2.2mg, 0.02mmol), the air in the reaction flask was replaced with a three-way connection with a high-purity oxygen balloon, and placed under oxygen conditions , in an oil bath at 50°C, the reaction was stirred until the raw materials disappeared, the solvent was directly distilled off under reduced pressure, and the residue was directly purified by column chromatography to obtain pure product 1. 1 H NMR (400MHz, CDCl 3 )δ8.04(d, J=8.3Hz, 1H), 7.90(d, J=8.4Hz, 1H), 7.70(t, J=7.7Hz, 4H), 7.59(d, J=7.7Hz, 1H) ,7.47(td,J=8.2,1.1Hz,2H),7.23(t,J=7.4Hz,2H),7.19–7.06(m,3H),7.03(d,J=8.3Hz,1H),6.80( t, J=7.4Hz, 1H), 3.85(d, J=14.1Hz, 1H), 3.47(d, J=14.1Hz, 1H); 13 C NMR (101MHz, CDCl ...
Embodiment 2
[0057] Preparation of 3-methyl-2-phenyl-2-(2-quinolylmethyl)indolin-3-one:
[0058] With reference to the method of embodiment 1. 11 H NMR (400MHz, CDCl 3 )δ8.05(dd, J=11.9,6.8Hz,2H),7.90(d,J=8.4Hz,1H),7.70(dd,J=7.4,5.5Hz,5H),7.49(q,J=7.7 Hz, 2H), 7.31(dd, J=8.0, 6.4Hz, 2H), 7.23(t, J=7.5Hz, 2H), 7.15(t, J=7.3Hz, 1H), 7.09(d, J=8.4 Hz, 2H), 6.85(d, J=8.2Hz, 1H), 6.54(d, J=7.2Hz, 1H), 3.85(d, J=14.1Hz, 1H), 3.46(d, J=14.1Hz, 1H),2.55(s,3H); 13 C NMR (101MHz, CDCl 3 )δ202.65,161.46,158.14,147.55,140.77,139.17,137.02,136.53,129.76,128.96,128.43,127.79,127.43,126.97,126.40,125.93,123.17,122.22,120.64,110.42,71.50,46.23,18.45。
Embodiment 3
[0060] Preparation of 5-methyl-2-phenyl-2-(2-quinolylmethyl)indolin-3-one:
[0061] With reference to the method of embodiment 1. 1 H NMR (400MHz, CDCl 3 )δ8.03(d, J=8.5Hz, 1H), 7.87(d, J=8.4Hz, 1H), 7.72–7.62(m, 4H), 7.47(d, J=7.5Hz, 1H), 7.35( s,1H),7.28–7.18(m,3H),7.14(t,J=7.2Hz,1H),7.07(d,J=8.4Hz,1H),7.00–6.88(m,2H),3.81(d ,J=14.0Hz,1H),3.47(d,J=14.0Hz,1H),2.25(s,3H); 13 C NMR (101MHz, CDCl 3 )δ202.27,159.44,157.98,147.53,139.12,139.07,136.56,129.75,128.88,128.65,128.42,127.79,127.45,126.98,126.38,125.91,124.69,123.15,120.00,113.17,72.01,46.26,20.70。
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com